JC virus small tumor antigen promotes S phase entry and cell cycle progression
- PMID: 39586476
- PMCID: PMC11647237
- DOI: 10.1016/j.tvr.2024.200298
JC virus small tumor antigen promotes S phase entry and cell cycle progression
Abstract
The early coding region of JC virus (JCV) encodes several regulatory proteins including large T antigen (LT-Ag), small t antigen (Sm t-Ag) and T' proteins because of the alternative splicing of the pre-mRNA. LT-Ag plays a critical role in cell transformation by targeting the key cell cycle regulatory proteins including p53 and pRb, however, the role of Sm t-Ag in this process remains elusive. Here, we investigated the effect of Sm t-Ag on the cell cycle progression and demonstrated that it facilitates S phase entry and exit when cells are released from G0/G1 growth arrest. Examination of the cell cycle stage specific expression profiles of the selected cyclins and cyclin-dependent kinases, including those active at the G1/S and G2/M transition state, demonstrated a higher level of early expression of these regulators such as cyclin B, cycling E, and Cdk2. In addition, analysis of the effect of Sm t-Ag on the growth promoting pathways including those active in the PI3K/Akt/mTOR axis showed substantially higher levels of the phosphorylated-Akt, -Gsk3-β and -S6K1 in Sm t-Ag-positive cells. Collectively, our results demonstrate that Sm t-Ag promotes cell cycle progression by activating the growth promoting pathways through which it may contribute to LT-Ag-mediated cell transformation.
Keywords: BK virus; Cancer; Cell cycle; JC virus; Merkel cell carcinoma virus; Papillomavirus; Polyomavirus; Transformation.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Authors declare no conflict of interest.
Figures
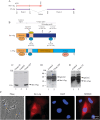

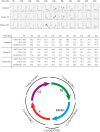



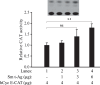
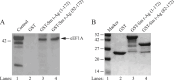

References
-
- J.S. Butel. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis 21 (200) 405-426. - PubMed
-
- Chen W., Hahn W.C. SV40 early region oncoproteins and human cell transformation. Histol. Histopathol. 2003;18:541–550. - PubMed
-
- Major E.O., Ault G.S. Progressive multifocal leukoencephalopathy: clinical and laboratory observations on a viral induced demyelinating disease in the immunodeficient patient. Curr. Opin. Neurol. 1995:184–190. - PubMed
-
- Frisque R.J., White F.A. In: Molecular Neurovirology, Pathogenesis of Viral CNS Infections. Roose R.P., editor. Humana Press Inc.; Totowa, NJ: 1992. The molecular biology of JC virus, causative agent of progressive multifocal leukoencophalopathy, p 25-158.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

