The cystogenic effects of ouabain in autosomal dominant polycystic kidney disease require cell caveolae
- PMID: 39586486
- PMCID: PMC12186285
- DOI: 10.1016/j.yexcr.2024.114356
The cystogenic effects of ouabain in autosomal dominant polycystic kidney disease require cell caveolae
Abstract
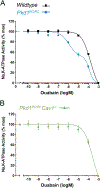
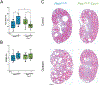
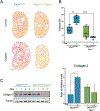
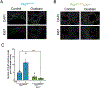
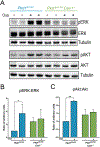
We have previously shown that the hormone ouabain is a circulating factor which can accelerate the progression of autosomal dominant polycystic kidney disease (ADPKD). At physiologic concentrations, ouabain increases cyst area and fibrosis in kidneys from ADPKD but not wildtype mice. These effects are due to an increased affinity for ouabain by its receptor, Na,K-ATPase (NKA), in the kidneys of ADPKD mice which leads to over-activation of NKA signaling function. Previous studies suggested that ouabain's stimulation of NKA signal transduction is mediated by NKA located within cell caveolae. Here, we determined whether caveolae are involved in the ouabain-induced progression of ADPKD cysts. We generated an ADPKD mouse with a global knockout of the main structural component of caveolae, caveolin-1 (CAV1), which we confirmed lacks caveolae in the kidney. When given physiological amounts of ouabain for 5 months, Pkd1RC/RCCav1-/- mice did not exhibit any changes in cyst progression, contrasting with the Pkd1RC/RC mice which showed a significant increase in cystic area and kidney fibrosis. Also, measures of ouabain-induced cell proliferation, including the number of Ki67-positive nuclei and phosphorylation of the extracellular regulated kinase (ERK) and protein kinase B (Akt), did not increase in the Pkd1RC/RCCav1-/- mice compared with the Pkd1RC/RC mice. Moreover, the abnormally increased affinity for ouabain of NKA in Pkd1RC/RC mice was restored to wildtype levels in the Pkd1RC/RCCav1-/- mice. This work highlights the role of caveolae in ouabain-induced NKA signaling and ADPKD cyst progression.
Keywords: Autosomal dominant polycystic kidney disease; Caveolae; Na,K-ATPase.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

