A Genetically Encoded Redox-Active Nicotinamide Amino Acid
- PMID: 39586687
- PMCID: PMC11797079
- DOI: 10.1021/acs.biochem.4c00530
A Genetically Encoded Redox-Active Nicotinamide Amino Acid
Abstract
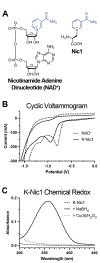
Nicotinamide-containing cofactors play an essential role in many enzymes that catalyze two-electron redox reactions. However, it is difficult to engineer nicotinamide binding sites into proteins due to the extended nature of the cofactor-protein interface and the precise orientation of the nicotinamide moiety required for efficient electron transfer to or from the substrate. To address these challenges, we genetically encoded a noncanonical amino acid (ncAA) bearing a nicotinamide side chain in bacteria. This redox-active amino acid, termed Nic1, exhibits similar electrochemical properties to the natural cofactor nicotinamide adenine dinucleotide (NAD+). Nic1 can be reversibly reduced and oxidized using chemical reagents both free in solution and when incorporated into a model protein. This genetically encodable cofactor can be introduced into proteins in a site-specific fashion and may serve as a tool to study electron-transfer mechanisms in enzymes and to engineer redox-active proteins.
Figures


Similar articles
-
RNA aptamers that bind flavin and nicotinamide redox cofactors.J Am Chem Soc. 1995 Feb 1;117(4):1246-57. doi: 10.1021/ja00109a008. J Am Chem Soc. 1995. PMID: 11539282
-
A Magnesium Binding Site And The Anomeric Effect Regulate The Abiotic Redox Chemistry Of Nicotinamide Nucleotides.Chemistry. 2024 Jun 20;30(35):e202400411. doi: 10.1002/chem.202400411. Epub 2024 May 23. Chemistry. 2024. PMID: 38640109
-
The Auxiliary NADH Dehydrogenase Plays a Crucial Role in Redox Homeostasis of Nicotinamide Cofactors in the Absence of the Periplasmic Oxidation System in Gluconobacter oxydans NBRC3293.Appl Environ Microbiol. 2021 Jan 4;87(2):e02155-20. doi: 10.1128/AEM.02155-20. Print 2021 Jan 4. Appl Environ Microbiol. 2021. PMID: 33127815 Free PMC article.
-
Nicotinamide Riboside: What It Takes to Incorporate It into RNA.Molecules. 2024 Aug 10;29(16):3788. doi: 10.3390/molecules29163788. Molecules. 2024. PMID: 39202867 Free PMC article. Review.
-
Engineering natural and noncanonical nicotinamide cofactor-dependent enzymes: design principles and technology development.Curr Opin Biotechnol. 2020 Dec;66:217-226. doi: 10.1016/j.copbio.2020.08.005. Epub 2020 Sep 18. Curr Opin Biotechnol. 2020. PMID: 32956903 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

