Sinomenine Ameliorated Microglial Activation and Neuropathic Pain After Chronic Constriction Injury Via TGF-β1/ALK5/Smad3 Signalling Pathway
- PMID: 39586784
- PMCID: PMC11588427
- DOI: 10.1111/jcmm.70214
Sinomenine Ameliorated Microglial Activation and Neuropathic Pain After Chronic Constriction Injury Via TGF-β1/ALK5/Smad3 Signalling Pathway
Abstract
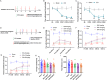
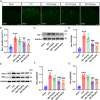
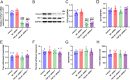
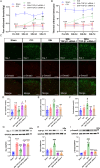
Sinomenine (SIN), a bioactive isoquinoline alkaloid extracted from the roots and stems of Sinomenium acutum, is efficacious against various chronic pain conditions. Inhibition of microglial activation at the spinal level contributes to the analgesic effects of SIN. Microglial activation in the spinal dorsal horn is key to sensitising neuropathic pain. Consequently, this study aimed to investigate whether the antinociceptive effects of SIN in neuropathic pain are induced through microglial inhibition and the underlying mechanisms. In this study, we observed that SIN alleviated chronic constriction injury (CCI)-induced pain hypersensitivity, spinal microglial activation and neuroinflammation. Consistently, SIN evoked the upregulation of transforming growth factor-beta1 (TGF-β1) and phosphorylated Smad3 in the L4-6 ipsilateral spinal dorsal horn of CCI mice. Intrathecal injection of TGF-β1 siRNA and an activin receptor-like receptor (ALK5) inhibitor reversed SIN's antinociceptive and antimicroglial effects on CCI mice. Moreover, targeting Smad3 in vitro with siRNA dampened the inhibitory effect of TGF-β1 on lipopolysaccharide-induced microglial activation. Finally, targeting Smad3 abrogated SIN-induced pain relief and microglial inhibition in CCI mice. These findings indicate that the TGF-β1/ALK5/Smad3 axis plays a key role in the antinociceptive effects of SIN on neuropathic pain, indicating its suppressive ability on microglia.
Keywords: Smad3; activin receptor–like receptor 5; microglia; neuropathic pain; sinomenine; transforming growth factor‐β1.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Bouhassira D., “Neuropathic Pain: Definition, Assessment and Epidemiology,” Revue Neurologique 175, no. 1–2 (2019): 16–25. - PubMed
-
- Alles S. R. A. and Smith P. A., “Etiology and Pharmacology of Neuropathic Pain,” Pharmacological Reviews 70, no. 2 (2018): 315–347. - PubMed
-
- Finnerup N. B., Kuner R., and Jensen T. S., “Neuropathic Pain: From Mechanisms to Treatment,” Physiological Reviews 101, no. 1 (2021): 259–301. - PubMed
-
- Kuner R., “Central Mechanisms of Pathological Pain,” Nature Medicine 16, no. 11 (2010): 1258–1266. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

