GSK3 inhibition improves skeletal muscle function and whole-body metabolism in male mouse models of Duchenne muscular dystrophy
- PMID: 39587049
- PMCID: PMC11589163
- DOI: 10.1038/s41467-024-53886-y
GSK3 inhibition improves skeletal muscle function and whole-body metabolism in male mouse models of Duchenne muscular dystrophy
Abstract
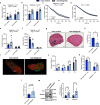
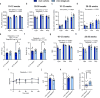
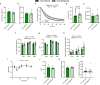
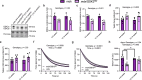
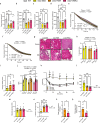
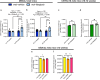
Inhibiting glycogen synthase kinase 3 (GSK3) improves muscle function, metabolism, and bone health in many diseases and conditions; however, whether GSK3 should be targeted for Duchenne muscular dystrophy (DMD), a severe muscle wasting disorder with no cure, remains unknown. Here, we show the effects of GSK3 inhibition in male DBA/2J (D2) and C57BL/10 (C57) mdx mice. Treating D2 mdx mice with GSK3 inhibitors alone or in combination with aerobic exercise improves muscle strength, endurance, and morphology, attenuates the hypermetabolic phenotype, and enhances insulin sensitivity. GSK3 inhibition in C57 mdx mice also improves muscle fatigue resistance and increases cage ambulation. Moreover, muscle-specific GSK3 knockdown in mdx mice augments muscle force production and endurance. In both mdx strains, GSK3 inhibition increases bone mineral content and density. Overall, these improvements to muscle, metabolic, and bone health with GSK3 inhibition in mdx mice may have clinical implications for patients with DMD, where the current standard of care, glucocorticoids, delay the loss of ambulation but increase the risk for insulin resistance and osteoporosis. Along with our observation of lowered β-catenin content in DMD myoblasts, a known cellular target for GSK3, this study provides ample evidence in support of inhibiting GSK3 for this disease.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests. A portion of this project, specifically the aged (28–30 weeks) tideglusib experiments, was funded by AMO-Pharma in partnership with a Brock-Niagara Validation Prototyping and Manufacturing Applied Project Grant (1:2 monetary ratio). AMO-Pharma also provided the tideglusib for the aged tideglusib experiments. For all other experiments, tideglusib was purchased from Sigma Alrdich (SML0339).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

