Autoantibodies immuno-mechanically modulate platelet contractile force and bleeding risk
- PMID: 39587073
- PMCID: PMC11589161
- DOI: 10.1038/s41467-024-54309-8
Autoantibodies immuno-mechanically modulate platelet contractile force and bleeding risk
Abstract
Altered mechanotransduction has been proposed as a putative mechanism for disease pathophysiology, yet evidence remains scarce. Here we introduce a concept we call single cell immuno-mechanical modulation, which links immunology, integrin biology, cellular mechanics, and disease pathophysiology and symptomology. Using a micropatterned hydrogel-laden coverslip compatible with standard fluorescence microscopy, we conduct a clinical mechanobiology study, specifically focusing on immune thrombocytopenia (ITP), an autoantibody-mediated platelet disorder that currently lacks a reliable biomarker for bleeding risk. We discover that in pediatric ITP patients (n = 53), low single platelet contraction force alone is a "physics-based" biomarker of bleeding (92.3% sensitivity, 90% specificity). Mechanistically, autoantibodies and monoclonal antibodies drive increases and decreases of cell force by stabilizing integrins in different conformations depending on the targeted epitope. Hence, immuno-mechanical modulation demonstrates how antibodies may pathologically alter mechanotransduction to cause clinical symptoms and this phenomenon can be leveraged to control cellular mechanics for research, diagnostic, and therapeutic purposes.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
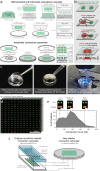



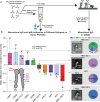


References
Publication types
MeSH terms
Substances
Grants and funding
- R35HL145000/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01HL155330/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- F31HL160210/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- F31 HL160210/HL/NHLBI NIH HHS/United States
- K25 HL141636/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

