Microglial cannabinoid receptor type II stimulation improves cognitive impairment and neuroinflammation in Alzheimer's disease mice by controlling astrocyte activation
- PMID: 39587077
- PMCID: PMC11589152
- DOI: 10.1038/s41419-024-07249-6
Microglial cannabinoid receptor type II stimulation improves cognitive impairment and neuroinflammation in Alzheimer's disease mice by controlling astrocyte activation
Abstract
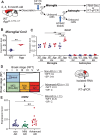
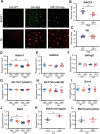
Alzheimer's disease (AD) is the most common form of dementia and is characterized by the accumulation of amyloid β (Aβ) and phosphorylated tau. Neuroinflammation, mainly mediated by glial activation, plays an important role in AD progression. Although there is growing evidence for the anti-neuroinflammatory and neuroprotective effects of the cannabinoid system modulation, the detailed mechanism remains unclear. To address these issues, we analyzed the expression levels of cannabinoid receptor type II (Cnr2/Cb2) in AppNL-G-F/NL-G-F mice and human AD precuneus, which is vulnerable to amyloid deposition in AD, and the effects of JWH 133, a selective CB2 agonist, on neuroinflammation in primary glial cells and neuroinflammation and cognitive impairment in AppNL-G-F/NL-G-F mice. The levels of Cnr2/Cb2 were upregulated in microglia isolated from the cerebral cortex of AppNL-G-F/NL-G-F mice. CNR2 expression was also increased in RNAs derived from human precuneus with advanced AD pathology. Chronic oral administration of JWH 133 significantly ameliorated the cognitive impairment of AppNL-G-F/NL-G-F mice without neuropsychiatric side effects. Microglia and astrocyte mRNAs were directly isolated from the mouse cerebral cortex by magnetic-activated cell sorting, and the gene expression was determined by quantitative PCR. JWH 133 administration significantly decreased reactive astrocyte markers and microglial C1q, an inducer for the reactive astrocytes in AppNL-G-F/NL-G-F mice. In addition, JWH133 administration inhibited the expression of p-STAT3 (signal transducer and activator of transcription 3) in astrocytes in AppNL-G-F/NL-G-F mice. Furthermore, JWH 133 administration suppressed dystrophic presynaptic terminals surrounding amyloid plaques. In conclusion, stimulation of microglial CB2 ameliorates cognitive dysfunction in AppNL-G-F/NL-G-F mice by controlling astrocyte activation and inducing beneficial neuroinflammation, and our study has implications that CB2 may represent an attractive therapeutic target for the treatment of AD and perhaps other neurodegenerative diseases involving neuroinflammation.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The experiments using human brains were approved by the Ethics Committees at Nagoya University (approval number #328) and Tokyo Metropolitan Institute (approval number # R21-145). Informed consent was obtained from their families. The experiments using genetically modified mice were approved by the Animal Care and Use Committee and the recombinant DNA experiment committee of Nagoya University (approval numbers R240025 and R240026, and #143, respectively). All procedures were conducted in accordance with the Declaration of Helsinki.
Figures




References
-
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 2023,19:1598–695. - PubMed
MeSH terms
Substances
Grants and funding
- JP24wm0425014/Japan Agency for Medical Research and Development (AMED)
- JP21wm0425019/Japan Agency for Medical Research and Development (AMED)
- JP21wm0425019/Japan Agency for Medical Research and Development (AMED)
- None/Takeda Science Foundation
- None/Hori Sciences and Arts Foundation (Hori Sciences & Arts Foundation)
- None/Hori Sciences and Arts Foundation (Hori Sciences & Arts Foundation)
- JPMJM2024/MEXT | Japan Science and Technology Agency (JST)
- JP23K14687/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22H04923/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22H04923/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

