Structural mechanisms of human sodium-coupled high-affinity choline transporter CHT1
- PMID: 39587078
- PMCID: PMC11589582
- DOI: 10.1038/s41421-024-00731-7
Structural mechanisms of human sodium-coupled high-affinity choline transporter CHT1
Abstract
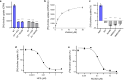
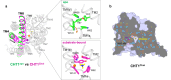
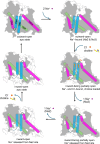
Mammalian sodium-coupled high-affinity choline transporter CHT1 uptakes choline in cholinergic neurons for acetylcholine synthesis and plays a critical role in cholinergic neurotransmission. Here, we present the high-resolution cryo-EM structures of human CHT1 in apo, substrate- and ion-bound, hemicholinium-3-inhibited, and ML352-inhibited states. These structures represent three distinct conformational states, elucidating the structural basis of the CHT1-mediated choline uptake mechanism. Three ion-binding sites, two for Na+ and one for Cl-, are unambiguously defined in the structures, demonstrating that both ions are indispensable cofactors for high-affinity choline-binding and are likely transported together with the substrate in a 2:1:1 stoichiometry. The two inhibitor-bound CHT1 structures reveal two distinct inhibitory mechanisms and provide a potential structural platform for designing therapeutic drugs to manipulate cholinergic neuron activity. Combined with the functional analysis, this study provides a comprehensive view of the structural mechanisms underlying substrate specificity, substrate/ion co-transport, and drug inhibition of a physiologically important symporter.
© 2024. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures




References
Grants and funding
- I-1578/Welch Foundation
- RP170644/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
- RP170644/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
- 32371300/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

