Ad26.RSV.preF completely protects calves from severe respiratory disease induced by bovine RSV challenge
- PMID: 39587114
- PMCID: PMC11589129
- DOI: 10.1038/s41541-024-01024-6
Ad26.RSV.preF completely protects calves from severe respiratory disease induced by bovine RSV challenge
Abstract
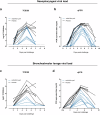
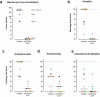
Vaccination with Ad26.RSV.preF, an Adenoviral serotype 26 vector encoding RSV F protein stabilized in its prefusion conformation, has previously shown to be immunogenic and protective in RSV seropositive adults and immunogenic in seropositive infants. Human and bovine RSV (bRSV) are genetically highly related and share many aspects of pathogenesis, epidemiology and clinical manifestations at young age. As such, infection of calves with bRSV represents a clinically relevant model with high translational value, enabling preclinical evaluation of Ad26.RSV.preF vaccine efficacy in seronegative young animals. Immunization of young calves with Ad26.RSV.preF induced antibodies neutralizing both human and bovine RSV as well as RSV-specific cellular responses. After bRSV challenge, placebo immunized calves showed viral replication in the respiratory tract, and developed fever and lethargy accompanied with severe respiratory distress, resulting in pre-termination of 7/8 calves. In contrast, all Ad26.RSV.preF immunized calves completed the study with only mild clinical symptoms, strongly and significantly diminished viral loads in nasopharynx and lungs, and only minimal lung pathology. Thus, Ad26.RSV.preF is immunogenic in young calves and efficacious in a stringent heterologous bRSV challenge model, demonstrating induction of broadly protective immunity against severe disease.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Authors K.D., M.H.-v.d.M., L.T., J.S., M.v.S., B.C., R.Z., and L.v.d.F. are (former) employees of Janssen Infectious Diseases & Vaccines and may own stock or stock options in Johnson & Johnson, its parent company, but declare no non-financial competing interests. All other authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

