Multimodal beneficial effects of BNN27, a nerve growth factor synthetic mimetic, in the 5xFAD mouse model of Alzheimer's disease
- PMID: 39587294
- PMCID: PMC12092300
- DOI: 10.1038/s41380-024-02833-w
Multimodal beneficial effects of BNN27, a nerve growth factor synthetic mimetic, in the 5xFAD mouse model of Alzheimer's disease
Abstract
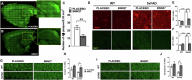
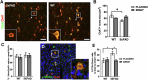
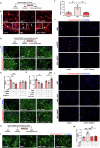
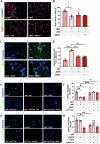
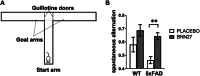
Alzheimer's Disease (AD) is an incurable and debilitating progressive, neurodegenerative disorder which is the leading cause of dementia worldwide. Neuropathologically, AD is characterized by the accumulation of Aβ amyloid plaques in the microenvironment of brain cells and neurovascular walls, chronic neuroinflammation, resulting in neuronal and synaptic loss, myelin and axonal failure, as well as significant reduction in adult hippocampal neurogenesis. The hippocampal formation is particularly vulnerable to this degenerative process, due to early dysfunction of the cholinergic circuit. Neurotrophic factors consist major regulatory molecules and their decline in AD is considered as an important cause of disease onset and progression. Novel pharmacological approaches are targeting the downstream pathways controlled by neurotrophins, such as nerve growth factor (NGF) receptors, TrkA and p75NTR, which enhance hippocampal neurogenic capacity and neuroprotective mechanisms, and potentially counteract the neurotoxic effects of amyloid deposition. BNN27 is a non-toxic, newly developed 17-spiro-steroid analog, penetrating the blood-brain-barrier (BBB) and mimicking the neuroprotective effects of NGF, acting as selective activator of its receptors, both TrkA and p75NTR, thus promoting survival of various neuronal cell types. Our present research aims at determining whether and which aspects of the AD-related pathology, BNN27 is able to alleviate, exploring the cellular and molecular AD components and link these changes with improvements in the cognitive performance of an animal AD model, the 5xFAD mice. Our results clearly indicate that BNN27 administration significantly reduced amyloid-β load in whole brain of the animals, enhanced adult hippocampal neurogenesis, restored cholinergic function and synaptogenesis, reducing inflammatory activation and leading to significant restoration of cognitive functions. BNN27 may represent a new lead multimodal molecule with neuroprotective, neurogenic and anti-neuroinflammatory actions for developing druggable anti-Alzheimeric agents. Proteomics data are available via ProteomeXchange with the identifier PXD044699.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: All authors, except Achille Gravanis, declare that they have not any competing financial interests in relation to the work described. Dr Achille Gravanis is the co-founder of spin-off Bionature EA LTD, proprietary of compound BNN27 (patented with the WO 2008/ 1555 34 A2 number at the World Intellectual Property Organization). Gravanis A is co-founder of Bionature E.A. Ltd. The BNN compounds are proprietary and patented by the Bionature E.A. Ltd ( http://www.bionature.net ) (Patent Number: WO2008/155534 A2). Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of FORTH (protocol code 262272 and date of approval 29 October 2018, FORTH Institute animal license: EL91-BIObr-01 and EL91-BIOexp-02). All primary cells derived from animals that were grouped housed in the Animal House of the Institute of Molecular Biology and Biotechnology (IMBB-FoRTH, Heraklion, Greece), in a temperature-controlled facility on a 12-h light/dark cycle, fed by standard chow diet and water ad libitum. All research activities strictly adhered to the EU adopted Directive 2010/63/EU on the protection of animals used for scientific purposes. All procedures were performed under the approval of Veterinary Directorate of Prefecture of Heraklion (Crete) and carried out in compliance with Greek Government guidelines and the guidelines of FORTH ethics committee and were performed in accordance with approved protocols from the Federation of European Laboratory Animal Science Associations (FELASA) and Use of Laboratory animals [License number: EL91-BIOexp-02), Approval Code: 360667, Approval Date: 29/11/2021 (active for 3 years)].
Figures


References
-
- World Alzheimer Report 2022: The Global Economic Impact of Dementia. London, UK: Alzheimer’s Disease International.
-
- Wilson DM 3rd, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186:693–714. - PubMed
-
- Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–90. - PubMed
-
- Arendt T, Bigl V, Tennstedt A, Arendt A. Correlation between cortical plaque count and neuronal loss in the nucleus basalis in Alzheimer's disease. Neuroscience Letters. 1984;48:81–85. - PubMed
MeSH terms
Substances
Grants and funding
- 101099145/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 765704/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

