ZW sex chromosome structure in Amborella trichopoda
- PMID: 39587314
- PMCID: PMC11649558
- DOI: 10.1038/s41477-024-01858-x
ZW sex chromosome structure in Amborella trichopoda
Abstract
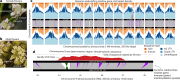
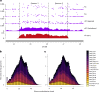
Sex chromosomes have evolved hundreds of times across the flowering plant tree of life; their recent origins in some members of this clade can shed light on the early consequences of suppressed recombination, a crucial step in sex chromosome evolution. Amborella trichopoda, the sole species of a lineage that is sister to all other extant flowering plants, is dioecious with a young ZW sex determination system. Here we present a haplotype-resolved genome assembly, including highly contiguous assemblies of the Z and W chromosomes. We identify a ~3-megabase sex-determination region (SDR) captured in two strata that includes a ~300-kilobase inversion that is enriched with repetitive sequences and contains a homologue of the Arabidopsis METHYLTHIOADENOSINE NUCLEOSIDASE (MTN1-2) genes, which are known to be involved in fertility. However, the remainder of the SDR does not show patterns typically found in non-recombining SDRs, such as repeat accumulation and gene loss. These findings are consistent with the hypothesis that dioecy is derived in Amborella and the sex chromosome pair has not significantly degenerated.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Renner, S. S. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot.101, 1588–1596 (2014). - PubMed
-
- Renner, S. S. & Müller, N. A. Plant sex chromosomes defy evolutionary models of expanding recombination suppression and genetic degeneration. Nat. Plants7, 392–402 (2021). - PubMed
-
- Soltis, P. S., Soltis, D. E. & Chase, M. W. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature402, 402–404 (1999). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

