Intrathecal lactate dehydrogenase A inhibitors FX11 and oxamate alleviate chronic constriction injury-induced nociceptive sensitization through neuroinflammation and angiogenesis
- PMID: 39587478
- PMCID: PMC11590346
- DOI: 10.1186/s10194-024-01916-x
Intrathecal lactate dehydrogenase A inhibitors FX11 and oxamate alleviate chronic constriction injury-induced nociceptive sensitization through neuroinflammation and angiogenesis
Abstract
Background: Neuropathic pain involves neuroinflammation and upregulation of glycolysis in the central nervous system. Unfortunately, few effective treatments are available for managing this type of pain. The overactivation of lactate dehydrogenase A (LDHA), an essential enzyme in glycolysis, can cause neuroinflammation and nociception. This study investigated the spinal role of LDHA in neuropathic pain.
Method: Using immunohistochemical analysis, nociceptive behavior, and western blotting, we evaluated the cellular mechanisms of intrathecal administration of LDHA inhibitors, including FX11 and oxamate, in chronic constriction injury (CCI)-induced neuropathic rats.
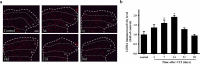
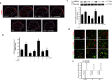
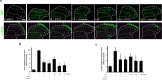
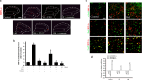
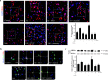
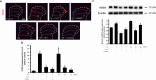
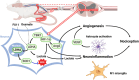
Result: FX11 and oxamate attenuated CCI-induced neuronal LDHA upregulation and nociceptive sensitization. Moreover, CCI-induced neuroinflammation, microglial polarization, and angiogenesis were attenuated by LDHA inhibitors. These inhibitors regulate the TANK binding kinase-1 (TBK1)/hypoxia-inducible factor 1 subunit alpha (HIF-1α) axis, crucial for controlling inflammation and new blood vessel growth. Additionally, CCI-induced nuclear LDHA translocation, as associated with oxidative stress resistance, was attenuated by LDHA inhibitors.
Conclusion: In conclusion, LDHA may be a potential therapeutic target for treating neuropathic pain by regulating neuroinflammation and angiogenesis.
Keywords: Angiogenesis; Lactate dehydrogenase A; Neuroinflammation; Neuropathic pain.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experimental procedures were approved by the National Sun Yat-sen University Institutional Committee for the Care and Use of Animals (Approval No. IACUC-10830 and 11137). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Ghazisaeidi S, Muley MM, Salter MW (2023) Neuropathic Pain: Mechanisms, Sex Differences, and Potential Therapies for a Global Problem. Annu Rev Pharmacol Toxicol 63:565–583 - PubMed
-
- Balanaser M et al (2023) Combination pharmacotherapy for the treatment of neuropathic pain in adults: systematic review and meta-analysis. Pain 164(2):230–251 - PubMed
MeSH terms
Substances
Grants and funding
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- NSTC112-2314-B-075-072/National Science and Technology Council
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
- V113C-056/Taipei Veterans General Hospital, Taiwan
LinkOut - more resources
Full Text Sources
Miscellaneous

