The impact of dexamethasone on short- and long-term mortality in hospitalized COVID-19 patients: a retrospective study
- PMID: 39587481
- PMCID: PMC11590211
- DOI: 10.1186/s12879-024-10216-3
The impact of dexamethasone on short- and long-term mortality in hospitalized COVID-19 patients: a retrospective study
Abstract
Background: Dexamethasone has been widely used in treating severe COVID-19 patients due to its anti-inflammatory properties. However, its long-term impact on mortality remain unclear.
Objective: To evaluate the effect of dexamethasone on short-term (28-day) and long-term (1-year) mortality in hospitalized COVID-19 patients and to explore its efficacy across different respiratory support.
Methods: A retrospective cohort study was conducted using the MIMIC-IV (v3.0) database. A total of 576 confirmed COVID-19 patients were included, with 288 patients receiving dexamethasone and 288 not receiving it, matched by propensity scores. Survival analyses assessed the impact of dexamethasone on mortality, and subgroup analyses were performed based on the type of respiratory support received.
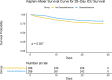
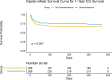
Results: After propensity score matching, dexamethasone treatment was associated with reduced mortality at both 28 days (adjusted HR 0.67, 95% CI 0.46-0.99, P = 0.045) and 1 year (adjusted HR 0.66, 95% CI 0.47-0.92, P = 0.014). Subgroup analysis revealed differential treatment effects by respiratory support type (P for interaction = 0.001 at 28 days and 0.004 at 1 year). The survival benefit was most pronounced in patients receiving NIV (28-day adjusted HR 0.15, 95% CI 0.05-0.42, P < 0.001) and significant in those receiving IMV (28-day adjusted HR 0.62, 95% CI 0.39-0.99, P = 0.045), while no significant benefit was observed in patients receiving oxygen therapy alone.
Conclusion: This retrospective study suggests that dexamethasone treatment was associated with reduced mortality in hospitalized COVID-19 patients, particularly in those receiving NIV or IMV. These findings add to the evidence supporting dexamethasone use in severe COVID-19 patients requiring respiratory support.
Keywords: COVID-19; Dexamethasone; MIMIC-IV; Mortality; Respiratory support; Retrospective study.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The MIMIC-IV database was approved by the Institutional Review Boards of Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA). Informed consent was obtained for the original data collection. The requirement for individual patient consent was waived as the project did not impact clinical care and all protected health information was de-identified. Consent for publication: Non-applicable. Competing interests: The authors declare no competing interests. Clinical trial registration: Non-applicable.
Figures
References
-
- E. C. WHO Coronavirus Disease (COVID-19) dashboard. BPJ. 2020;10.
-
- Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report. medRxiv. 2020.
-
- Popa-Fotea N. Dexamethasone in Hospitalized Patients with Covid-19. 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical