Efficacy and safety of human umbilical cord-derived mesenchymal stem cells versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease: a multicentre, randomized, double-blind, phase 2 trial
- PMID: 39587570
- PMCID: PMC11590523
- DOI: 10.1186/s12916-024-03782-5
Efficacy and safety of human umbilical cord-derived mesenchymal stem cells versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease: a multicentre, randomized, double-blind, phase 2 trial
Abstract
Background: Failure of systemic corticosteroid therapy is common in patients with newly diagnosed acute graft-versus-host disease (aGVHD) above grade II. Mesenchymal stem cells (MSCs) have been used as a tolerable and potentially effective second-line therapy for steroid-refractory aGVHD (SR-aGVHD); however, well-designed, prospective, controlled studies are lacking.
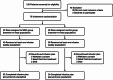
Methods: This multicentre, randomized, double-blind, placebo-controlled, exploratory phase 2 study enrolled patients with SR-aGVHD above grade II from 7 centres. Patients were randomized 1:1 to receive umbilical cord-derived MSCs or placebo added to one centre's choice of second-line agents (except for ruxolitinib). The agents were infused twice weekly. Patients with complete response (CR), no response (NR), or progression of disease (PD) at d28 received 8 infusions, and those with partial response (PR) received the above infusions for another 4 weeks. The per-protocol population consisted of patients who received ≥ 8 infusions. The primary endpoint was the overall response rate (ORR, CR + PR) at d28, analyzed in the per-protocol and intention-to-treat populations.
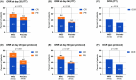
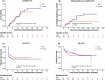
Results: Seventy-eight patients (median age 38, range 13-62) were enrolled: 40 in the MSC group and 38 in the control. Patients in the MSC group received a median of 8 doses, with a median response time of 14 days. In intention-to-treat analysis, ORR at d28 was 60% for MSC group and 50% for control group (p = 0.375). The 2-year cumulative incidence of moderate to severe cGVHD was marginally lower in the MSC group than in the control (13.8% vs. 39.8%, p = 0.067). The 2-year failure-free survival was similar between the MSC and control groups (52.5% vs. 44.4%, p = 0.43). In per-protocol analysis (n = 62), ORR at d28 was significantly greater in the MSC group than in the control group (71.9% vs. 46.7%, p = 0.043). Among patients with gut involvement, ORR at d28 was significantly greater in the MSC group than in the control (66.7% vs. 33.3%, p = 0.031). The adverse events incidences were similar between groups.
Conclusions: In this exploratory study, there was no superior ORR at d28 demonstrated in the MSC group compared with the control. However, MSCs showed a gradual treatment effect at a median of 2 weeks. Patients who completed 8 infusions may benefit from adding MSCs to one conventional second-line agent, especially those with gut involvement. MSCs was well tolerated in patients with SR-aGVHD.
Trial registration: chictr.org.cn ChiCTR2000035740.
Keywords: Acute graft-versus-host disease; Haematopoietic stem cell transplantation; Mesenchymal stem cells; Steroid-refractory.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Medical Ethics Committee of the General Hospital of the Chinese People’s Liberation Army (C2020-016–05). Patient’s written consent to participate in this clinical trial was obtained prior to any study-specific procedures. Consent for publication: Not applicable. Competing interests: X.H. is an employee of Platinumlife Biotechnology (Beijing). The other authors have no conflicts of interest to report.
Figures
References
-
- Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7(2):e157–67. - DOI - PubMed
-
- Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11(2):e147–59. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

