Inhaled antibiotics for treating pneumonia in invasively ventilated patients in intensive care unit: a meta-analysis of randomized clinical trials with trial sequential analysis
- PMID: 39587607
- PMCID: PMC11587605
- DOI: 10.1186/s13054-024-05159-9
Inhaled antibiotics for treating pneumonia in invasively ventilated patients in intensive care unit: a meta-analysis of randomized clinical trials with trial sequential analysis
Abstract
Background: The use of inhaled antibiotics for treating pneumonia in invasively ventilated patients offers a direct approach, allowing for high local concentrations of the drug in the lower respiratory tract while simultaneously reducing systemic toxicity. However, the real efficacy and safety of nebulized antibiotics remain unclear. The aim of the present is to assess among critically adult patients with pneumonia and invasive ventilation, whether receiving adjuvant inhaled antibiotics improves the rate of microbiological eradication.
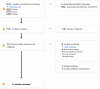
Methods: A comprehensive literature search of randomized clinical trials (RCTs) was conducted (from inception until September 20, 2024, PROSPERO-CRD592906) across Medline, Embase, and Scopus. Randomized controlled trials, enrolling intensive care units (ICU) patients with pneumonia and comparing nebulized antimicrobial therapy (inhaled group) with intravenous antimicrobial treatment or intravenous antimicrobial therapy plus inhaled placebo (control group), were included. The primary outcome was the rate of microbiological eradication after treatment. Secondary outcomes were the rate of clinical recovery, the incidence of drug-related adverse events, ICU and hospital mortality. A qualitative analysis was conducted according to the GRADE framework. Data were pooled using an odds-ratio analysis. The heterogeneity and reliability of our results were evaluated using the I2-statistic and trial sequential analysis (TSA), respectively.
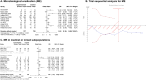
Results: A total of 11 RCTs (1472 patients) met the inclusion criteria. Compared to controls, the use of adjuvant inhaled antibiotics determined a greater rate of microbiological eradication (OR 2.63, 95% CI 1.36-5.09; low certainty of evidence). The TSA confirmed the reliability of our primary outcome. Moreover, nebulized antibiotics increased the risk of bronchospasm (OR 3.15, 95% CI 1.33-7.47; high evidence), while nephrotoxicity, clinical recovery, ICU and hospital survival (either in the case of pneumonia caused by MDR bacteria or not) were not different between groups.
Conclusions: In conclusion, compared to the sole intravenous therapy, the use of adjuvant inhaled antibiotics for treatment of pneumonia in invasively ventilated critically ill patients was associated with a greater incidence of microbiological eradication (low GRADE and high risk of publication bias), but not with clinical recovery and survival.
Keywords: Antibiotics; Infection; Inhaled; Multi-drug resistant; Multi-drug resistant organism; Nebulized; Treatment.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

