High cell density cultivation by anaerobic respiration
- PMID: 39587616
- PMCID: PMC11590539
- DOI: 10.1186/s12934-024-02595-8
High cell density cultivation by anaerobic respiration
Abstract
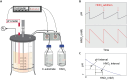
Background: Oxygen provision is a bottleneck in conventional aerobic high cell density culturing (HCDC) of bacteria due to the low O2 solubility in water. An alternative could be denitrification: anaerobic respiration using nitrogen oxides as terminal electron acceptors. Denitrification is attractive because NO3- is soluble in water, the end-product (N2) is harmless, and denitrification is widespread among bacteria, hence suitable organisms for most purposes can be found. The pH must be controlled by injection of an inorganic acid to compensate for the pH increase by NO3--consumption, resulting in salt accumulation if feeding the bioreactor with NO3- salt. We avoid this with our novel pH-stat approach, where the reactor is supplied with 5 M HNO3 to compensate for the alkalization, thus sustaining NO3--concentration at a level determined by the pH setpoint. Here we present the first feasibility study of this method, growing the model strain Paracoccus denitrificans anaerobically to high densities with glucose as the sole C-source and NO3- as the N-source and electron acceptor.
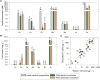
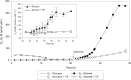
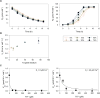
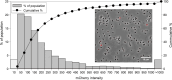
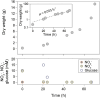
Results: Our fed-batch culture reached 20 g cell dry weight L-1, albeit with slower growth rates than observed in low cell density batch cultures. We explored reasons for slow growth, and the measured trace element uptake indicates it is not a limiting factor. Bioassays with spent medium excluded accumulation of inhibitory compounds at high cell density as the reason for the slow growth. The most plausible reason is that high metabolic activity led to CO2/H2CO3 accumulation, thus suppressing pH, leading to a paucity in HNO3-feeding until N2-sparging had removed sufficient CO2. The three free intermediates in the denitrification pathway (NO3- → NO2- → NO → N2O → N2) can all reach toxic concentrations if the electron flow is unbalanced, and this did occur if cells were glucose-limited. On the other hand, accumulation of polyhydroxyalkanoates occurred if the cells were NO3--limited. Carefully balancing glucose provision according to the HNO3 injected is thus crucial.
Conclusions: This work provides a proof of concept, while also identifying CO2/H2CO3 accumulation as a hurdle that must be overcome for further development and optimization of the method.
Keywords: Denitrification; High cell density cultivation; pH–stat.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: A patent application, describing the basic concept of HCDC by denitrification has been filed (European Patent App No. 22700061.9 and US Patent App. 18/270,538, 2024).
Figures






References
-
- UN Population Division. World population prospects 2022: Summary of results. UN DESA/POP/2022/TR/NO. 3. 2022.
-
- Westhoek H, Rood T, Van den Berg M, Janse J, Nijdam D, Reudink M, et al. The protein puzzle. Hague: PBL Netherlands Environmental Assessment Agency; 2011. p. 221.
-
- Suman G, Nupur M, Anuradha S, Pradeep B. Single cell protein production: a review. Int J Curr Microbiol App Sci. 2015;4(9):251–62.
-
- Spalvins K, Zihare L, Blumberga D. Single cell protein production from waste biomass: comparison of various industrial by-products. Energy Proc. 2018;147:409–18. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

