The Parkinson's disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons
- PMID: 39587654
- PMCID: PMC11587650
- DOI: 10.1186/s13024-024-00779-9
The Parkinson's disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons
Erratum in
-
Correction: The Parkinson's disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons.Mol Neurodegener. 2024 Dec 18;19(1):94. doi: 10.1186/s13024-024-00791-z. Mol Neurodegener. 2024. PMID: 39696367 Free PMC article. No abstract available.
Abstract
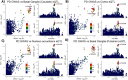
Background: Variants in the CTSB gene encoding the lysosomal hydrolase cathepsin B (catB) are associated with increased risk of Parkinson's disease (PD). However, neither the specific CTSB variants driving these associations nor the functional pathways that link catB to PD pathogenesis have been characterized. CatB activity contributes to lysosomal protein degradation and regulates signaling processes involved in autophagy and lysosome biogenesis. Previous in vitro studies have found that catB can cleave monomeric and fibrillar alpha-synuclein, a key protein involved in the pathogenesis of PD that accumulates in the brains of PD patients. However, truncated synuclein isoforms generated by catB cleavage have an increased propensity to aggregate. Thus, catB activity could potentially contribute to lysosomal degradation and clearance of pathogenic alpha synuclein from the cell, but also has the potential of enhancing synuclein pathology by generating aggregation-prone truncations. Therefore, the mechanisms linking catB to PD pathophysiology remain to be clarified.
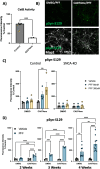
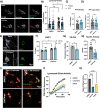
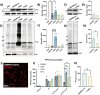
Methods: Here, we conducted genetic analyses of the association between common and rare CTSB variants and risk of PD. We then used genetic and pharmacological approaches to manipulate catB expression and function in cell lines, induced pluripotent stem cell-derived dopaminergic neurons and midbrain organoids and assessed lysosomal activity and the handling of aggregated synuclein fibrils.
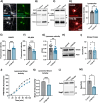
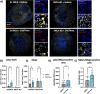
Results: We find that catB inhibition impairs autophagy, reduces glucocerebrosidase (encoded by GBA1) activity, and leads to an accumulation of lysosomal content. In cell lines, reduction of CTSB gene expression impairs the degradation of pre-formed alpha-synuclein fibrils, whereas CTSB gene activation enhances fibril clearance. In midbrain organoids and dopaminergic neurons treated with alpha-synuclein fibrils, catB inhibition potentiates the formation of inclusions which stain positively for phosphorylated alpha-synuclein.
Conclusions: These results indicate that the reduction of catB function negatively impacts lysosomal pathways associated with PD pathogenesis, while conversely catB activation could promote the clearance of pathogenic alpha-synuclein.
© 2024. The Author(s).
Figures



Update of
-
The Parkinson's disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons.bioRxiv [Preprint]. 2023 Nov 15:2023.11.11.566693. doi: 10.1101/2023.11.11.566693. bioRxiv. 2023. Update in: Mol Neurodegener. 2024 Nov 25;19(1):88. doi: 10.1186/s13024-024-00779-9. PMID: 38014143 Free PMC article. Updated. Preprint.
-
The Parkinson's disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons.Res Sq [Preprint]. 2024 Mar 19:rs.3.rs-3979098. doi: 10.21203/rs.3.rs-3979098/v1. Res Sq. 2024. Update in: Mol Neurodegener. 2024 Nov 25;19(1):88. doi: 10.1186/s13024-024-00779-9. PMID: 38562709 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

