Computerized decision support to optimally funnel patients through the diagnostic pathway for dementia
- PMID: 39587679
- PMCID: PMC11590510
- DOI: 10.1186/s13195-024-01614-5
Computerized decision support to optimally funnel patients through the diagnostic pathway for dementia
Abstract
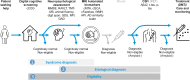
Background: The increasing prevalence of dementia and the introduction of disease-modifying therapies (DMTs) highlight the need for efficient diagnostic pathways in memory clinics. We present a data-driven approach to efficiently guide stepwise diagnostic testing for three clinical scenarios: 1) syndrome diagnosis, 2) etiological diagnosis, and 3) eligibility for DMT.
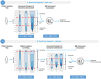
Methods: We used data from two memory clinic cohorts (ADC, PredictND), including 504 patients with dementia (302 Alzheimer's disease, 107 frontotemporal dementia, 35 vascular dementia, 60 dementia with Lewy bodies), 191 patients with mild cognitive impairment, and 188 cognitively normal controls (CN). Tests included digital cognitive screening (cCOG), neuropsychological and functional assessment (NP), MRI with automated quantification, and CSF biomarkers. Sequential testing followed a predetermined order, guided by diagnostic certainty. Diagnostic certainty was determined using a clinical decision support system (CDSS) that generates a disease state index (DSI, 0-1), indicating the probability of the syndrome diagnosis or underlying etiology. Diagnosis was confirmed if the DSI exceeded a predefined threshold based on sensitivity/specificity cutoffs relevant to each clinical scenario. Diagnostic accuracy and the need for additional testing were assessed at each step.
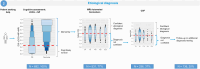
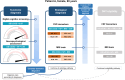
Results: Using cCOG as a prescreener for 1) syndrome diagnosis has the potential to accurately reduce the need for extensive NP (42%), resulting in syndrome diagnosis in all patients, with a diagnostic accuracy of 0.71, which was comparable to using NP alone. For 2) etiological diagnosis, stepwise testing resulted in an etiological diagnosis in 80% of patients with a diagnostic accuracy of 0.77, with MRI needed in 77%, and CSF in 37%. When 3) determining DMT eligibility, stepwise testing (100% cCOG, 83% NP, 75% MRI) selected 60% of the patients for confirmatory CSF testing and eventually identified 90% of the potentially eligible patients with AD dementia.
Conclusions: Different diagnostic pathways are accurate and efficient depending on the setting. As such, a data-driven tool holds promise for assisting clinicians in selecting tests of added value across different clinical contexts. This becomes especially important with DMT availability, where the need for more efficient diagnostic pathways is crucial to maintain the accessibility and affordability of dementia diagnoses.
Keywords: Alzheimer’s disease; Data-driven diagnosis; Differential diagnosis; Eligibility.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The data in this study were collected during routine care and retrieved retrospectively, which was approved by the Medical Ethical Committee (METc) of the VUmc Medical Center and at the local clinics in the PredictND study. All patients provided written informed consent for their data to be used for research purposes. Consent for publication: Not applicable. Competing interests: HR performed contract research for Combinostics; all funding is paid to her institution. JL is a shareholder at Combinostics and Combinostics holds three patents regarding its DSI technology. Research programs of Wiesje van der Flier have been funded by ZonMW, NWO, EU-JPND, EU-IHI, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc, Novartis-NL, Life-MI, AVID, Roche BV, Fujifilm, Eisai, Combinostics. WF holds the Pasman chair. WF is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). WF has been an invited speaker at Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape), NovoNordisk, Springer Healthcare, European Brain Council. WF is consultant to Oxford Health Policy Forum CIC, Roche, Biogen MA Inc, and Eisai. WF is member of steering cie of NovoNordisk evoke/evoke+. WF participated in advisory boards of Biogen MA Inc, Roche, and Eli Lilly. WF is member of the steering committee of EVOKE/EVOKE+ (NovoNordisk). All funding is paid to her institution. WF is member of the steering committee of PAVE, and Think Brain Health. WF was associate editor of Alzheimer, Research & Therapy in 2020/2021. WF is associate editor at Brain. FB is steering committee or Data Safety Monitoring Board member for Biogen, Merck, Eisai and Prothena. FB is advisory board member for Combinostics, Scottish Brain Sciences. Consultant for Roche, Celltrion, Rewind Therapeutics, Merck, Bracco. FB has research agreements with ADDI, Merck, Biogen, GE Healthcare, Roche. FB is co-founder and shareholder of Queen Square Analytics LTD. All other authors report no financial disclosures or conflicts of interest.
Figures

References
-
- 2016 Alzheimer's disease facts and figures. Alzheimers Dement, 2016. 12(4): p. 459-509. - PubMed
-
- van Maurik, I.S., et al., A more precise diagnosis by means of amyloid PET contributes to delayed institutionalization, lower mortality, and reduced care costs in a tertiary memory clinic setting. Alzheimer's & Dementia, 2022. n/a(n/a). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

