[Establishment of a population pharmacokinetic model for linezolid in neonates with sepsis]
- PMID: 39587744
- PMCID: PMC11601107
- DOI: 10.7499/j.issn.1008-8830.2406078
[Establishment of a population pharmacokinetic model for linezolid in neonates with sepsis]
Abstract
Objectives: To establish the pharmacokinetic model of linezolid in neonates, and to optimize the administration regimen.
Methods: A prospective study was conducted among 64 neonates with sepsis who received linezolid as anti-infective therapy, and liquid chromatography-tandem mass spectrometry was used to measure the plasma concentration of the drug. Clinical data were collected, and nonlinear mixed effects modeling was used to establish a population pharmacokinetic (PPK) model. Monte Carlo simulation and evaluation was performed for the optimal administration regimen of children with different features.
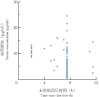
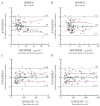
Results: The pharmacokinetic properties of linezolid in neonates could be described by a single-compartment model with primary elimination, and the population typical values for apparent volume of distribution and clearance rate were 0.79 L and 0.34 L/h, respectively. The results of goodness of fit, visualization verification, and the Bootstrap method showed that the model was robust with reliable results of parameter estimation and prediction. Monte Carlo simulation results showed that the optimal administration regimen for linezolid in neonates was as follows: 6 mg/kg, q8h, at 28 weeks of gestational age (GA); 8 mg/kg, q8h, at 32 weeks of GA; 9 mg/kg, q8h, at 34-37 weeks of GA; 11 mg/kg, q8h, at 40 weeks of GA.
Conclusions: The PPK model established in this study can provide a reference for individual administration of linezolid in neonates. GA and body weight at the time of administration are significant influencing factors for the clearance rate of linezolid in neonates.
目的: 建立利奈唑胺在新生儿中的药动学模型并优化给药方案。方法: 前瞻性收集64例使用利奈唑胺抗感染治疗的败血症新生儿为研究对象,采用液相色谱串联质谱法检测血药浓度,收集临床资料,采用非线性混合效应建模法建立群体药动学(population pharmacokinetic, PPK)模型,采用蒙特卡洛模拟和评价不同特征患儿的最佳给药方案。结果: 利奈唑胺在新生儿体内的药动学特性可用一个具有一级消除的单室模型来描述,表观分布容积和清除率的群体典型值分别为0.79 L和0.34 L/h。拟合优度、可视化验证及自举法结果表明,模型稳健,参数估算及预测结果可靠。蒙特卡洛模拟显示新生儿利奈唑胺最佳给药方案,胎龄(gestational age, GA)28周6 mg/kg,q8h;GA 32周8 mg/kg,q8h;GA 34~37周9 mg/kg,q8h;GA 40周11 mg/kg,q8h。结论: 该研究建立的PPK模型可为新生儿利奈唑胺的个体化给药提供参考,GA和用药时体重是影响新生儿利奈唑胺清除率的显著性因素。.
Keywords: Linezolid; Monte Carlo simulation; Neonate; Population pharmacokinetics; Sepsis; Therapeutic drug monitoring.
Conflict of interest statement
所有作者均声明不存在利益冲突。
Figures

References
-
- Nelson JD, Bradley JS, Kimberlin DW, et al. . 2021 Nelsons Pediatric Antimicrobial Therapy[M]. 27th ed. Itasca: American Academy of Pediatrics, 2021.
-
- Liu P, Capitano B, Stein A, et al. . Clinical outcomes of linezolid and vancomycin in patients with nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus stratified by baseline renal function: a retrospective, cohort analysis[J]. BMC Nephrol, 2017, 18(1): 168. DOI: 10.1186/s12882-017-0581-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

