Comparison of Tegoprazan and Lansoprazole in Patients With Erosive Esophagitis up to 4 Weeks: A Multi-Center, Randomized, Double-Blind, Active-Comparator Phase 4 Trial
- PMID: 39587796
- PMCID: PMC11650551
- DOI: 10.1111/nmo.14969
Comparison of Tegoprazan and Lansoprazole in Patients With Erosive Esophagitis up to 4 Weeks: A Multi-Center, Randomized, Double-Blind, Active-Comparator Phase 4 Trial
Abstract
Background: The aims of this study were to confirm the non-inferiority of tegoprazan to lansoprazole up to week 4 in patients with erosive esophagitis (EE) and to evaluate its effectiveness in rapid mucosal healing and symptom relief at week 2.
Methods: In this multi-center, randomized, double-blind, active-comparator non-inferiority trial, 218 patients with endoscopically confirmed EE (Los Angeles Classification Grades A-D) were randomly allocated to either the tegoprazan (50 mg) or lansoprazole (30 mg) group. The primary endpoint was the cumulative proportion of patients with healed EE up to week 4, as confirmed through endoscopy. The proportion of patients with healed EE at week 2 was also evaluated. Furthermore, CYP2C19 genotypes, symptoms, safety, and tolerability were assessed.
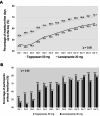
Key results: In the full-analysis set, 103 and 109 participants in the tegoprazan and lansoprazole groups, respectively, were analyzed. The cumulative healing rates up to week 4 were 95.2% (98/103) and 86.2% (94/109) (difference [95% confidence interval], 8.91 [1.22-16.59]; p < 0.0001 for non-inferiority and 0.0266 for superiority), while those at week 2 were 88.4% (91/103) and 82.6% (90/109) (5.78 [-3.66-15.22], p = 0.0005 for non-inferiority) for tegoprazan and lansoprazole, respectively. Tegoprazan showed consistent healing rates regardless of CYP2C19 genotypes.
Conclusions and inferences: Tegoprazan was superior to lansoprazole in the treatment of EE up to 4 weeks. Further studies are necessary to confirm these findings and clarify the superiority of tegoprazan, especially in the treatment of severe EE.
Trial registration: ClinicalTrials.gov identifier: NCT05267743.
Keywords: erosive esophagitis; lansoprazole; potassium competitive acid blocker; proton pump inhibitor; tegoprazan.
© 2024 The Author(s). Neurogastroenterology & Motility published by John Wiley & Sons Ltd.
Conflict of interest statement
Declaration of personal interests: Ah Rong Kim, Hye Won Kim, Kyu Hoon Gee, Hyun Wook Park, and Geun Seog Song are employees of HK inno.N Corp., Seoul, Korea.
The authors declare no conflicts of interest.
Figures

References
-
- Lee S. J., Song C. W., Jeen Y. T., et al., “Prevalence of Endoscopic Reflux Esophagitis Among Koreans,” Journal of Gastroenterology and Hepatology 16 (2001): 373–376. - PubMed
-
- Kim J. I., Kim S. G., Kim N., et al., “Changing Prevalence of Upper Gastrointestinal Disease in 28 893 Koreans From 1995 to 2005,” European Journal of Gastroenterology & Hepatology 21 (2009): 787–793. - PubMed
-
- Park C. H., Kim K. O., Baek I. H., et al., “Differences in the Risk Factors of Reflux Esophagitis According to Age in Korea,” Diseases of the Esophagus 27 (2014): 116–121. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

