Immunogenicity of dupilumab in adult and pediatric patients with atopic dermatitis
- PMID: 39588375
- PMCID: PMC11586717
- DOI: 10.3389/fimmu.2024.1466372
Immunogenicity of dupilumab in adult and pediatric patients with atopic dermatitis
Abstract
Background: Development of anti-drug antibodies (ADAs) and neutralizing antibodies (NAbs) to monoclonal antibodies may adversely impact pharmacokinetics, efficacy, and/or safety.
Objective: To describe incidence, titer, and persistence of dupilumab ADAs and NAbs, and their effects on pharmacokinetics, efficacy, and safety in patients with atopic dermatitis (AD).
Methods: This analysis included seven phase 3 randomized, placebo-controlled (N=2,992) and two long-term open-label extension (N=2,287) trials of subcutaneous dupilumab in adults and pediatric patients with moderate-to-severe AD. ADA, NAb, and dupilumab concentration in serum were assessed using validated immunoassays. ADA impacts on efficacy (EASI) and safety were assessed.
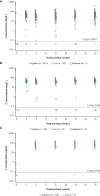
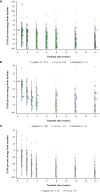
Results: Treatment-emergent ADAs were observed in up to 8.6% (aged ≥18 years), 16.0% (12-17 years), 5.3% (6-11 years), and 2.0% (6 months to 5 years) dupilumab-treated patients. Among dupilumab-treated patients, ≤3.7% had persistent responses, <1% had high titers (≥10,000), and ≤5.1% were NAb-positive. NAbs were more common in patients with moderate- and high-titer ADA responses. High-titer ADAs, while infrequent, were the variable most associated with lower dupilumab concentrations in serum and loss of efficacy, independent of NAb status. Efficacy was generally similar in ADA-positive and -negative patients. For most patients with high- or moderate-titer ADAs, titers decreased and efficacy improved over time with continued dupilumab treatment. ADA-positive and -negative patients had similar incidences of treatment-emergent and serious treatment-emergent adverse events. One patient with high-titer ADAs developed serum sickness.
Conclusion: In patients with AD, ADAs and NAbs had minimal impact on dupilumab concentration, efficacy, and safety, except for high-titer ADAs in a small number of patients.
Clinical trial registration: ClinicalTrials.gov, identifiers (NCT02277743, NCT02277769, NCT02260986, NCT02395133, NCT01949311, NCT03054428, NCT03345914, NCT02612454, and NCT03346434).
Keywords: ADA; NAb; anti-drug antibody; atopic dermatitis; dupilumab; immunogenicity; neutralizing antibody.
Copyright © 2024 Kamal, Kosloski, Lai, Partridge, Rajadhyaksha, Kanamaluru, Bansal, Shabbir, Shumel, Ardeleanu, Richards, Yan, Xu, Rodríguez-Marco, Xiao, Khokhar, Gherardi, Babilonia, Maloney, Mortensen, Akinlade, Braunstein, Stahl, Torri, Davis and DiCioccio.
Conflict of interest statement
MAK, MPK, C-HL, MAP, AB, BS, MA, HY, JX, FAK, EB, JM, BA, NB, NS, AT, JDD, and ATD are employees and shareholders of Regeneron Pharmaceuticals Inc. MR, AS and EM are former employees and shareholders of Regeneron Pharmaceuticals Inc. VK, SMR, CRX, AR-M, and GG are employees of and may hold stock and/or stock options in Sanofi. The authors declare that this research was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. The funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
Figures

References
-
- van Schouwenburg PA, van de Stadt LA, de Jong RN, van Buren EEL, Kruithof S, de Groot E, et al. . Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralization. Ann Rheum Dis. (2013) 72:104–9. doi: 10.1136/annrheumdis-2012-201445 - DOI - PubMed
-
- Christen U, Thuerkauf R, Stevens R, Lesslauer W. Immune response to a recombinant human TNFR55-IgG1 fusion protein: auto-antibodies in rheumatoid arthritis (RA) and multiple sclerosis (MS) patients have neither neutralizing nor agonist activities. Hum Immunol. (1999) 60:774–90. doi: 10.1016/s0198-8859(99)00068-3 - DOI - PubMed
-
- Carlsson M, Braddock M, Li Y, Wang J, Xu W, White N, et al. . Evaluation of antibody properties and clinically relevant immunogenicity, anaphylaxis, and hypersensitivity reactions in two phase III trials of tralokinumab in severe, uncontrolled asthma. Drug Saf. (2019) 42:769–84. doi: 10.1007/s40264-018-00788-w - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

