Transcultural Validation of the Five-Item Dry Eye Questionnaire for Indonesian Populations
- PMID: 39588394
- PMCID: PMC11586872
- DOI: 10.7759/cureus.72288
Transcultural Validation of the Five-Item Dry Eye Questionnaire for Indonesian Populations
Abstract
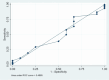
Purpose Indonesian dry eye (DE) disease (DED) prevalence data remain scarce, and Indonesian validations of the five-item Dry Eye Questionnaire (DEQ-5) are unavailable. We aimed to translate and validate an Indonesian language adaptation of the DEQ-5 (INDO-DEQ-5) for local populations. Methods Our observational study involved linguistic validation of the translation, reliability testing, dataset screening, and data collection through ophthalmic examinations and interviews. Outpatient adults with dry eye symptoms and formal DED diagnoses were included. Data were captured at patients' enrolment, at their self-administered INDO-DEQ-5, and at ophthalmic examinations. This data was statistically analyzed for agreement, test-retest reliability, and by a receiver operator characteristic (ROC) curve for dry eye sensitivity and specificity. Results Tear breakup time (TBUT) was the most frequently used clinical diagnostic parameter, with 96.8% of DED patients having abnormal TBUT. We captured 87.23% of all true DE cases, while the ROC and area under the ROC curve analyses found similar sensitivity between the INDO-DEQ-5 and the composite clinical diagnosis of DE, and TBUT was equivalent to the INDO-DEQ-5 in clinical diagnoses. The level of reliability of the INDO-DEQ-5 was moderate, and subscales were moderate or substantial. Conclusion The prediction of DE with TBUT was similar to predictions using the composite definition of clinical DE diagnosis, while the INDO-DEQ-5 diagnostic accuracy was similar to that of TBUT or a composite clinical diagnosis. Thus, the INDO-DEQ-5 is a reliable tool for the clinical evaluation and diagnosis of DE in Indonesian populations and will enable clinicians to rapidly collect accurate data to improve therapeutic management.
Keywords: deq-5; dry eye disease; language adaptation; tear film ocular surface society dry eye workshop; validation.
Copyright © 2024, Noor et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Medical and Health Research Ethics Committee of Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada issued approval N/A. This study followed the tenets of the Declaration of Helsinki. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: Funding was provided for manuscript writing support to Dr Shawna Tan, Medical Writers Asia. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- TFOS DEWS II pathophysiology report. Bron AJ, de Paiva CS, Chauhan SK, et al. https://doi.org/10.1016/j.jtos.2017.05.011. Ocul Surf. 2017;15:438–510. - PubMed
-
- TFOS DEWS II epidemiology report. Stapleton F, Alves M, Bunya VY, et al. The Ocular Surface. 2017;15:334–365. - PubMed
-
- Long-term incidence of dry eye in an older population. Moss SE, Klein R, Klein BE. Optom Vis Sci. 2008;85:668–674. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
