Radiation Chemistry Reveals the Reaction Mechanisms Involved in the Reduction of Vinylene Carbonate in the Solid Electrolyte Interphase of Lithium-Ion Batteries
- PMID: 39588750
- PMCID: PMC11997941
- DOI: 10.1002/cssc.202402091
Radiation Chemistry Reveals the Reaction Mechanisms Involved in the Reduction of Vinylene Carbonate in the Solid Electrolyte Interphase of Lithium-Ion Batteries
Abstract
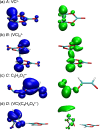
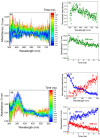
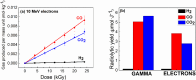
A safe and efficient lithium-ion battery requires including an additive in the electrolyte. Among the additives used, vinylene carbonate (VC) is particularly interesting, because it leads to the formation of a stable and protective solid electrolyte interphase (SEI) on the negative electrode. However, the reduction behavior of VC, resulting in polymer formation, is complex, and many questions remain as to the corresponding reaction mechanisms. In particular, in conventional battery studies, it is not possible to observe the transient species formed during reduction. Using picosecond pulsed radiolysis coupled with theoretical chemistry calculations, we showed that, once formed, the anion radical VC⋅- can undergo ring opening in a few nanoseconds or generate VC2⋅-. Within 100 ns, each of these anions then leads to the formation of VCC3H2O3⋅-. This latter species starts oligomerizing. Eventually, a polymer is formed. Although it mainly consists of poly(VC) units, other chemical functions, such as alkyl groups, are also present, which highlights the role played by water, even in trace amounts. Lastly, we propose a scheme of the reaction mechanisms involved in VC reduction, leading to its polymerization. Clearly, the polymer formed from VC at the SEI of lithium-ion batteries has a complex structure.
Keywords: Lithium-ion batteries; Radiolysis; Reaction mechanisms; Reactive intermediates; Vinylene carbonate.
© 2024 The Author(s). ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



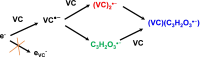



References
-
- Tarascon J. M., Armand M., Nature 2001, 414, 359–367. - PubMed
-
- Armand M., Tarascon J.-M., Nature 2008, 451, 652–657. - PubMed
-
- Jin Y., Kneusels N.-J. H., Marbella L. E., Castillo-Martínez E., Magusin P. C. M. M., Weatherup R. S., Jónsson E., Liu T., Paul S., Grey C. P., J. Am. Chem. Soc. 2018, 140, 9854–9867. - PubMed
-
- Martínez de la Hoz J. M., Balbuena P. B., Phys. Chem. Chem. Phys. 2014, 16, 17091–17098. - PubMed
-
- Xu C., Lindgren F., Philippe B., Gorgoi M., Björefors F., Edström K., Gustafsson T., Chem. Mater. 2015, 27, 2591–2599.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

