Bacterial Rps3 counters oxidative and UV stress by recognizing and processing AP-sites on mRNA via a novel mechanism
- PMID: 39588766
- PMCID: PMC11662941
- DOI: 10.1093/nar/gkae1130
Bacterial Rps3 counters oxidative and UV stress by recognizing and processing AP-sites on mRNA via a novel mechanism
Abstract
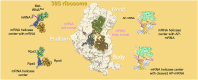
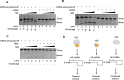
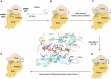
Lesions and stable secondary structures in mRNA severely impact the translation efficiency, causing ribosome stalling and collisions. Prokaryotic ribosomal proteins Rps3, Rps4 and Rps5, located in the mRNA entry tunnel, form the mRNA helicase center and unwind stable mRNA secondary structures during translation. However, the mechanism underlying the detection of lesions on translating mRNA is unclear. We used Cryo-EM, biochemical assays, and knockdown experiments to investigate the apurinic/apyrimidinic (AP) endoribonuclease activity of bacterial ribosomes on AP-site containing mRNA. Our biochemical assays show that Rps3, specifically the 130RR131 motif, is important for recognizing and performing the AP-endoribonuclease activity. Furthermore, structural analysis revealed cleaved mRNA product in the 30S ribosome entry tunnel. Additionally, knockdown studies in Mycobacterium tuberculosis confirmed the protective role of Rps3 against oxidative and UV stress. Overall, our results show that prokaryotic Rps3 recognizes and processes AP-sites on mRNA via a novel mechanism that is distinct from eukaryotes.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
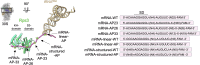






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

