Long-Term Adverse Effects of Perinatal Hypoxia on the Adult Pulmonary Circulation Vary Between Males and Females in a Murine Model
- PMID: 39589302
- PMCID: PMC11627267
- DOI: 10.33549/physiolres.935481
Long-Term Adverse Effects of Perinatal Hypoxia on the Adult Pulmonary Circulation Vary Between Males and Females in a Murine Model
Abstract
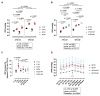
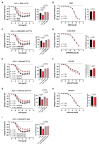
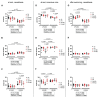
Adverse events during the perinatal period are associated with an increased risk to develop cardiometabolic diseases later in life. We established a murine model to study long-term effects of perinatal hypoxia (PH) on the pulmonary circulation. We previously demonstrated that PH led to an impaired regulation of pulmonary vascular tone in adulthood, linked to alterations in K+ channels in males and in the nitric oxide (NO)/cyclic guanosine monophosphate pathway in females. Moreover, simultaneous administration of inhaled NO (iNO) during PH exposure prevented adverse effects of PH on adult pulmonary vasculature in females. The present study showed that PH induced a significant increase in right ventricular pressure in males and females, and an enhanced sensitivity to acute hypoxia in females. PH significantly reduced acetylcholine-induced relaxation in pulmonary artery, to a greater extent in females than in males. PH led to right ventricular hypertrophy in adulthood, appearing earlier in males than in females. Morphometric measurements showed a significant increase in the number of 25-75-µm pulmonary vessels in male lungs following PH, probably resulting in increased pulmonary vascular resistance. The effects of prolonged hypoxia in adulthood differed between males and females. Perinatal iNO during PH prevented PH-induced alterations in the cardiopulmonary system, whereas perinatal iNO alone could have some adverse effects. Therefore, PH led to long-lasting alterations in the regulation of adult pulmonary circulation, which vary between males and females. In males, the increased pulmonary vascular resistance was associated with morphological changes besides functional alterations, whereas females showed an important pulmonary vascular dysfunction. Keywords: Perinatal hypoxia, Pulmonary circulation, Endothelium-dependent relaxation, Phosphodiesterases, Sex differences.
Conflict of interest statement
Figures