IL-6 and Cardiovascular Risk: A Narrative Review
- PMID: 39589436
- PMCID: PMC11599326
- DOI: 10.1007/s11883-024-01259-7
IL-6 and Cardiovascular Risk: A Narrative Review
Abstract
Purpose of review: The objective of this narrative review is to summarize data from recently published prospective observational studies that analyze the association between circulating interleukin-6 (IL-6) levels and cardiovascular clinical or imaging endpoints.
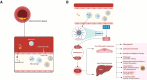
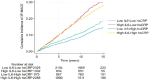
Recent findings: Higher levels of IL-6 are associated with a higher risk of cardiovascular death, major adverse cardiovascular events, myocardial infarction, stroke, peripheral artery disease, and heart failure. Imaging studies have also shown an association between IL-6 and carotid intima-media thickness progression, carotid plaque progression, severity, and vulnerability. These observations have been consistent across a wide range of study populations and after adjusting for traditional and emerging risk factors including high-sensitivity C-reactive protein. Robust epidemiologic evidence supports IL-6 as a central mediator of cardiovascular risk along with human genetic studies and mechanistic experiments. Ongoing clinical studies are testing the therapeutic hypothesis of IL-6 inhibition in patients with atherosclerotic cardiovascular disease or heart failure.
Keywords: Atherosclerosis; Cardiovascular outcomes; Hs-CRP; Inflammation; Interleukin-6; Risk prediction.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: NNM has served as a consultant for receiving and received grants/other payments from AbbVie, Celgene, Janssen Pharmaceuticals, Novartis, AMGEN, Astra Zeneca, Abcentra, Tourmaline, Bristol Meyers Squibb, Sun Pharmaceuticals and Celgene. EdG is an employee of Tourmaline Bio, Inc. MDS is supported by institutional grants from Amgen, Arrowhead, Boehringer Ingelheim, 89Bio, Esperion, Novartis, Ionis, Merck, New Amsterdam, and Cleerly. MDS has participated in Scientific Advisory Boards with Amgen, Agepha, Ionis, Novartis, New Amsterdam, and Merck. MDS has also served as a consultant for Ionis, Novartis, Regeneron, Aidoc, Shanghai Pharma Biotherapeutics, Kaneka, Novo Nordisk, Arrowhead, and Tourmaline. Human and Animal Rights and Informed Consent: No human or animal subjects by the authors were used in this study.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

