Effect of microplastic on sorption, toxicity, and mineralization of 2,4-dichlorophenoxyacetic acid ionic liquids
- PMID: 39589505
- PMCID: PMC11599340
- DOI: 10.1007/s00253-024-13353-6
Effect of microplastic on sorption, toxicity, and mineralization of 2,4-dichlorophenoxyacetic acid ionic liquids
Abstract
Recently, there has been significant focus on microplastics in the environment, especially regarding their role in sorption-desorption processes of emerging contaminants, impacting pollutant migration between aquatic and terrestrial ecosystems. Notably, the newest pollutants in such environments are the herbicide formulations known as ionic liquids (ILs), which integrate the structure of classic herbicidal anion with surface-active cations acting as an adjuvant. In our study, we synthesized herbicidal ILs by combining 2,4-D anion with cetyltrimethylammonium [CTA] and didecyldimethylammonium [DDA] cations. We investigated whether ILs and the mixture of salts, when exposed to polyethylene (PE) microplastics, differ in properties. We analyzed their sorption on defined PE particles, evaluated toxicity on Pseudomonas putida KT2440 using trans/cis ratio of unsaturated fatty acids, and assessed biodegradability with OECD 301F standard test. Results indicate IL cations and anions behave as distinct entities, questioning IL synthesis feasibility. Hydrophobic adjuvants were found to adsorb onto PE microplastic surfaces (5-60% [CTA] > [DDA]), posing potential threats of surface-active xenobiotic accumulation. This highlights the need to explore microplastics' role as sorbents of hazardous adjuvants in agriculture, potentially competing with humic acids and affecting xenobiotic bioavailability. Consequently, xenobiotics may persist longer in the environment, facilitated by microplastic mobility between aquatic and terrestrial ecosystems. KEY POINTS: • Microplastics act as sorbents, accumulating xenobiotics and limiting biodegradation. • Sorption of surfactant cations on microplastics reduces soil bacteria toxicity. • Research confirms independent action of ions from ionic liquids in the environment.
Keywords: Pseudomonas putida KT2440; 2,4-D; Antimicrobial activity; Herbicides; Polyethylene.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors. Conflict of interest: The authors declare no competing interests.
Figures

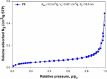
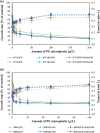
 ) and trans/cis ratio of unsaturated fatty acids (
) and trans/cis ratio of unsaturated fatty acids (
 ) of Pseudomonas putida KT2440 in the presence of PE microplastic. All compounds used were in EC50 concentrations determined within this study
) of Pseudomonas putida KT2440 in the presence of PE microplastic. All compounds used were in EC50 concentrations determined within this study
References
-
- Akanyange SN, Zhang Y, Zhao X, Adom-Asamoah G, Ature A-RA, Anning C, Tianpeng C, Zhao H, Lyu X, Crittenden JC (2022) A holistic assessment of microplastic ubiquitousness: pathway for source identification in the environment. Sustain Prod Consum 33:113–145. 10.1016/j.spc.2022.06.020
-
- Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52:1704–1724. 10.1021/acs.est.7b05559 - PubMed
-
- Appetecchi GB, Scaccia S, Tizzani C, Alessandrini F, Passerini S (2006) Synthesis of hydrophobic ionic liquids for electrochemical applications. J Electrochem Soc 153:A1685. 10.1149/1.2213420
-
- Arthur C, Baker J, Bamford H (2009) Proceedings of the International Research Workshop on the occurrence, effects, and fate of microplastic marine debris. National Oceanic and Atmospheric Administration
-
- Ateia M, Zheng T, Calace S, Tharayil N, Pilla S, Karanfil T (2020) Sorption behavior of real microplastics (MPs): insights for organic micropollutants adsorption on a large set of well-characterized MPs. Sci Total Environ 720:137634. 10.1016/j.scitotenv.2020.137634 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

