Succinate-producing microbiota drives tuft cell hyperplasia to protect against Clostridioides difficile
- PMID: 39589553
- PMCID: PMC11602550
- DOI: 10.1084/jem.20232055
Succinate-producing microbiota drives tuft cell hyperplasia to protect against Clostridioides difficile
Abstract
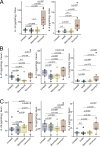
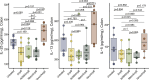
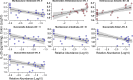
The role of microbes and their metabolites in modulating tuft cell (TC) dynamics in the large intestine and the relevance of this pathway to infections is unknown. Here, we uncover that microbiome-driven colonic TC hyperplasia protects against Clostridioides difficile infection. Using selective antibiotics, we demonstrate increased type 2 cytokines and TC hyperplasia in the colon but not in the ileum. We demonstrate the causal role of the microbiome in modulating this phenotype using fecal matter transplantation and administration of consortia of succinate-producing bacteria. Administration of succinate production-deficient microbes shows a reduced response in a Pou2f3-dependent manner despite similar intestinal colonization. Finally, antibiotic-treated mice prophylactically administered with succinate-producing bacteria show increased protection against C. difficile-induced morbidity and mortality. This effect is nullified in Pou2f3-/- mice, confirming that the protection occurs via the TC pathway. We propose that activation of TCs by the microbiota in the colon is a mechanism evolved by the host to counterbalance microbiome-derived cues that facilitate invasion by pathogens.
© 2024 Kellogg et al.
Conflict of interest statement
Disclosures: T.D. Kellogg reported a patent to US 63/626,305 pending “Microbiome engineering to induce colonic Tuft Cell expansions protects from
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

