Target-controlled dialysis for antibiotics (TCD-ABx)
- PMID: 39589615
- PMCID: PMC11599528
- DOI: 10.1186/s40635-024-00696-7
Target-controlled dialysis for antibiotics (TCD-ABx)
Abstract
Background: Effective antimicrobial therapy is an essential part of intensive care medicine and renal replacement therapy is an important and common intervention which significantly affects the pharmacokinetics of many antimicrobials. This is especially critical for substances with a narrow therapeutic range, creating a dilemma of weighing the risk of toxicity from increased drug exposure against risk of ineffective treatment and promotion of antimicrobial resistance. To address this problem, we investigate a target-controlled dialysis by in vitro experiments - a novel technique in which drug is spiked into the dialysis solution to make use of the physicochemical properties of renal replacement therapy for solute transport, with the goal to reduce the risk of inadequate drug exposure.
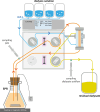
Methods: Five antibiotics (ceftazidime, meropenem, piperacillin/tazobactam, vancomycin, flucloxacillin) were dialyzed in an in vitro model of continuous veno-venous hemodialysis using 1 L of bovine serum albumin solution as simulated patient plasma compartment. This was done with and without antibiotics in target concentrations added to the dialysis solution, mimicking three clinically relevant scenarios: (i) target-controlled dialysis in a subject with sub-therapeutic drug levels, (ii) target-controlled dialysis in a subject with supra-therapeutic drug levels, and (iii) traditional dialysis of drugs starting at the target concentration. Drug levels were quantified by high-performance liquid chromatography. Additionally, the stability over 24 h of all antibiotics in two typical dialysis solutions was assessed.
Results: Our data shows that with target-controlled dialysis, antibiotic concentrations will change in the desired direction towards the target concentration, depending on the patients' unbound drug levels in relation to the concentration in the dialysis solution. The desired target concentrations can be induced and maintained, regardless of the initial concentration. Furthermore, the stability tests revealed only a minor and clinically irrelevant loss in drug concentration (all < 10.2%) after 12 h.
Conclusions: We outlined the mechanistic plausibility and provided experimental evidence of the feasibility of the target-controlled dialysis concept, which could help to maintain therapeutic concentrations of many time-dependent antibiotics in critically ill patients under renal replacement therapy. The required stability in dialysis solutions was shown for a set of important antibiotics. The next step will be the prudent application of this concept to patients in clinical trials.
Keywords: Antibiotics; Hemodialysis; Intensive care; Renal replacement therapy; Target-controlled dialysis; Therapeutic drug monitoring.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have no conflict of interest.
Figures





References
-
- Abdulla A, Ewoldt TMJ, Hunfeld NGM et al (2020) The effect of therapeutic drug monitoring of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients: the DOLPHIN trial protocol of a multi-centre randomised controlled trial. BMC Infect Dis 20:57. 10.1186/s12879-020-4781-x - PMC - PubMed
-
- Asín-Prieto E, Rodríguez-Gascón A, Isla A (2015) Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J Infect Chemother 21(5):319–329. 10.1016/j.jiac.2015.02.001 - PubMed
-
- Böhler J, Donauer J, Keller F (1999) Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int 56:S24–S28. 10.1046/j.1523-1755.56.s.72.2.x - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

