Nanoparticles in the battle against Candida auris biofilms: current advances and future prospects
- PMID: 39589626
- PMCID: PMC11968567
- DOI: 10.1007/s13346-024-01749-w
Nanoparticles in the battle against Candida auris biofilms: current advances and future prospects
Abstract
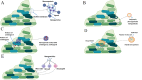
Candida auris has emerged as a significant global health threat due to its multidrug resistance and ability to form robust biofilms, particularly on medical devices and hospital surfaces. Biofilms protect C. auris from antifungal treatments and the host immune response, making infections persistent and difficult to control. This review explores the potential of nanoparticles to overcome the limitations of traditional antifungal therapies in combating C. auris biofilms. Nanoparticles, with their unique physicochemical properties, offer promising strategies to penetrate biofilm matrices, deliver antifungal agents, and disrupt biofilm structure. Various types of nanoparticles, including metallic, polymeric, lipid-based, and cyclodextrin-based, demonstrate enhanced biofilm penetration and antifungal activity. Their ability to generate reactive oxygen species, disrupt cell adhesion, and release antifungals in a controlled manner makes them ideal candidates for biofilm-targeted therapies. This review presents the current advancements in nanoparticle-based solutions, emphasizing the need for further research into their mechanisms of action, safety, and clinical application. By addressing the challenge of C. auris biofilms specifically, this review provides a critical synthesis of existing knowledge and identifies future directions for developing effective antifungal therapies using nanotechnology.
Keywords: Antifungal therapy; Biofilm; Multidrug resistance; Nanoparticles.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This article does not contain any studies with human participants or animals performed by any of the authors. Consent for publication: Not applicable. Competing interests: The authors have no relevant financial or nonfinancial interests to disclose.
Figures

References
-
- Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida Auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53(1):41–4. - PubMed
-
- Fayed B, Lazreg IK, AlHumaidi RB, Qasem MA, Alajmy BMGN, Bojbarah FM, et al. Intra-clade heterogeneity in Candida Auris: risk of management. Curr Microbiol. 2023;80(9):295. - PubMed
-
- Fayed B, Shakartalla SB, Sabbah H, Dalle H, Tannira M, Senok A, et al. Transcriptome Analysis of Human Dermal Cells Infected with Candida Auris Identified Unique Pathogenesis/Defensive mechanisms particularly ferroptosis. Mycopathologia. 2024;189(4):65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

