Use of Multiplex Molecular Panels to Diagnose Urinary Tract Infection in Older Adults
- PMID: 39589743
- PMCID: PMC11600226
- DOI: 10.1001/jamanetworkopen.2024.46842
Use of Multiplex Molecular Panels to Diagnose Urinary Tract Infection in Older Adults
Abstract
Importance: Multiplex molecular syndromic panels for diagnosis of urinary tract infection (UTI) lack clinical data supporting their use in routine clinical care. They also have the potential to exacerbate inappropriate antibiotic prescribing.
Objective: To describe the frequency of unspecified multiplex testing in administrative claims with a primary diagnosis of UTI in the Medicare population over time, to assess costs, and to characterize the health care professionals (eg, clinicians, laboratories, physician assistants, and nurse practitioners) and patient populations using these tests.
Design, setting, and participants: This cohort study used Centers for Medicare & Medicaid Services (CMS) claims data for Medicare beneficiaries. The study included older community-dwelling adults and nursing home residents with fee-for-service Medicare Part A and Part B benefits from January 1, 2016, to December 31, 2023.
Main outcomes and measures: Multiplex syndromic panels were identified using carrier claims (ie, claims for clinician office or laboratory services). The annual rate of claims was measured for multiplex syndromic panels with a primary diagnosis of UTI per 10 000 eligible Medicare beneficiaries. The performing and referring specialties of health care professionals listed on claims of interest and the proportion of claims that occurred among beneficiaries residing in a nursing home were described.
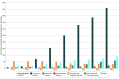
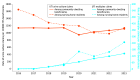
Results: Between 31 110 656 and 36 175 559 Medicare beneficiaries with fee-for-service coverage annually (2016-2023) were included in this study. In this period, 1 679 328 claims for UTI multiplex testing were identified. The median age of beneficiaries was 77 (IQR, 70-84) years; 34% of claims were from male beneficiaries and 66% were from female beneficiaries. From 2016 to 2023, the observed rate of UTI multiplex testing increased from 2.4 to 148.1 claims per 10 000 fee-for-service beneficiaries annually, and the proportion of claims that occurred among beneficiaries residing in a nursing home ranged from 1% in 2016 to 12% in 2020. In addition to laboratories or pathologists, urology was the most common clinician specialty conducting this testing. The CMS-assigned referring clinician specialty was most frequently urology or advanced practice clinician for claims among community-dwelling beneficiaries compared with internal medicine or family medicine for claims among nursing home residents. In 2023, the median cost of a multiplex test in the US was $585 (IQR, $516-$695 for Q1-Q3), which was more than 70 times higher than the median cost of $8 for a urine culture (IQR, $8-$16 for Q1-Q3).
Conclusions and relevance: This cohort study of Medicare beneficiaries with fee-for-service coverage from 2016 to 2023 found increasing use of emerging multiplex testing for UTI coupled with high costs to the Medicare program. Monitoring and research are needed to determine the effects of multiplex testing on antimicrobial use and whether there are clinical situations in which this testing may benefit patients.
Conflict of interest statement
Figures
Comment in
References
-
- List of microbial tests. US Food and Drug Administration . 2024. Accessed April 15, 2024. https://www.fda.gov/medical-devices/in-vitro-diagnostics/nucleic-acid-ba...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

