Ferumoxytol nanozymes effectively target chronic biofilm infections in apical periodontitis
- PMID: 39589820
- PMCID: PMC11785919
- DOI: 10.1172/JCI183576
Ferumoxytol nanozymes effectively target chronic biofilm infections in apical periodontitis
Abstract
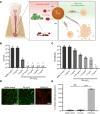
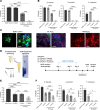
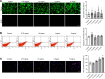
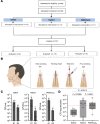
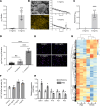
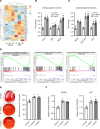
Bacterial biofilms are pervasive and recalcitrant to current antimicrobials, causing numerous infections. Iron oxide nanozymes, including an FDA-approved formulation, ferumoxytol (FMX), show potential against biofilm infections via catalytic activation of hydrogen peroxide (H2O2). However, clinical evidence regarding the efficacy and therapeutic mechanisms of FMX is lacking. Here, we investigate whether FMX nanozymes can treat chronic biofilm infections and compare their bioactivity to that of the gold standard sodium hypochlorite (NaOCl), a potent but caustic disinfectant. Clinical performance was assessed in patients with apical periodontitis, an intractable endodontic infection affecting half of the global adult population. Data show robust antibiofilm activity by a single application of FMX with H2O2 achieving results comparable to those seen with NaOCl without adverse effects. FMX binds efficiently to the bacterial pathogens Enterococcus faecalis and Fusobacterium nucleatum and remains catalytically active without being affected by dental tissues. This allows for effective eradication of endodontic biofilms via on-site free radical generation without inducing cytotoxicity. Unexpectedly, FMX promotes growth of stem cells of the apical papilla (SCAPs), with transcriptomic analyses revealing upregulation of proliferation-associated pathways and downregulation of cell cycle suppressor genes. Notably, FMX activates SCAP pluripotency and WNT/NOTCH signaling that induces its osteogenic capacity. Together, these results show that FMX nanozymes are clinically effective against severe chronic biofilm infection with pathogen targeting and unique stem cell-stimulatory properties, offering a regenerative approach to antimicrobial therapy.
Keywords: Bacterial infections; Drug therapy; Infectious disease; Nanotechnology.
Conflict of interest statement
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

