Staggered immunization with mRNA vaccines encoding SARS-CoV-2 polymerase or spike antigens broadens the T cell epitope repertoire
- PMID: 39589869
- PMCID: PMC11626164
- DOI: 10.1073/pnas.2406332121
Staggered immunization with mRNA vaccines encoding SARS-CoV-2 polymerase or spike antigens broadens the T cell epitope repertoire
Abstract
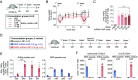
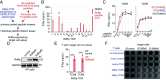
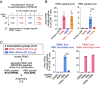
Combining a T cell-targeting mRNA vaccine encoding the conserved SARS-CoV-2 RNA-dependent RNA polymerase, RdRp, with a Spike-encoding mRNA vaccine may offer an additional pathway toward COVID-19 protection. Here, we show that a nucleoside-modified RdRp mRNA vaccine raises robust and durable CD8+ T cell responses in mice. Immunization drives a CD8+ T cell response enriched toward a specific RdRp epitope. Unexpectedly, coadministration of mRNA vaccines encoding RdRp or the Spike Receptor Binding Domain (RBD) dampens RBD-specific immune responses. Contralateral administration reduces the suppression of RBD-specific T cell responses while type I interferon signaling blockade restores RBD-specific antibodies. A staggered immunization strategy maintains both RBD vaccine-mediated antibody and T cell responses as well as protection against lethal SARS-CoV-2 challenge in human ACE2 transgenic mice. In HLA-A2.1 transgenic mice, the RdRp vaccine elicits CD8+ T cell responses against HLA-A*02:01-restricted epitopes recognized by human donor T cells. These results highlight RdRp as a candidate antigen for COVID-19 vaccines. The findings also offer insights into crafting effective multivalent mRNA vaccines to broaden CD8+ T cell responses against SARS-CoV-2 and potentially other viruses with pandemic potential.
Keywords: SARS-CoV-2; T cell receptor specificity; anti-pathogen T cell response; interferon signaling; mRNA vaccines.
Conflict of interest statement
Competing interests statement:The authors declare the following competing financial interest(s): C.G.R. is a co-founder of Sofie Biosciences and Trethera Corporation. He and the University of California hold equity in Sofie Biosciences and Trethera Corporation. O.N.W. currently has consulting, equity, and/or board relationships with Trethera Corporation, Kronos Biosciences, Sofie Biosciences, Breakthrough Properties, Vida Ventures, Nammi Therapeutics, Two River, Iconovir, Appia BioSciences, and Allogene Therapeutics. None of the companies listed above contributed to or directed any of the research reported in this article. Y.K.T. is an employee of Acuitas Therapeutics, a company involved in the development of lipid nanoparticle-encapsulated mRNA vaccines. Y.K.T. is named on patents that describe lipid nanoparticles for delivery of nucleic acids, including mRNA and the use of modified mRNA in lipid nanoparticles as a vaccine platform. N.P. is named on patents describing the use of nucleoside-modified mRNA in lipid nanoparticles as a vaccine platform. He has disclosed those interests fully to the University of Pennsylvania, and he has in place an approved plan for managing any potential conflicts arising from licensing of those patents. N.P. served on the mRNA strategic advisory board of Sanofi Pasteur in 2022. N.P. is a member of the Scientific Advisory Board of AldexChem.
Figures



References
-
- Muik A., et al. , Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T cell immunity. Cell Rep. 42, 112888 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

