PGC-1α drives small cell neuroendocrine cancer progression toward an ASCL1-expressing subtype with increased mitochondrial capacity
- PMID: 39589879
- PMCID: PMC11626175
- DOI: 10.1073/pnas.2416882121
PGC-1α drives small cell neuroendocrine cancer progression toward an ASCL1-expressing subtype with increased mitochondrial capacity
Abstract
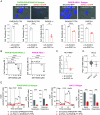
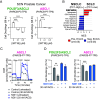
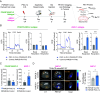
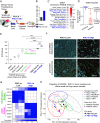
Adenocarcinomas from multiple tissues can converge to treatment-resistant small cell neuroendocrine (SCN) cancers composed of ASCL1, POU2F3, NEUROD1, and YAP1 subtypes. We investigated how mitochondrial metabolism influences SCN cancer (SCNC) progression. Extensive bioinformatics analyses encompassing thousands of patient tumors and human cancer cell lines uncovered enhanced expression of proliferator-activatedreceptor gamma coactivator 1-alpha (PGC-1α), a potent regulator of mitochondrial oxidative phosphorylation (OXPHOS), across several SCNCs. PGC-1α correlated tightly with increased expression of the lineage marker Achaete-scute homolog 1, (ASCL1) through a positive feedback mechanism. Analyses using a human prostate tissue-based SCN transformation system showed that the ASCL1 subtype has heightened PGC-1α expression and OXPHOS activity. PGC-1α inhibition diminished OXPHOS, reduced SCNC cell proliferation, and blocked SCN prostate tumor formation. Conversely, PGC-1α overexpression enhanced OXPHOS, validated by small-animal Positron Emission Tomography mitochondrial imaging, tripled the SCN prostate tumor formation rate, and promoted commitment to the ASCL1 lineage. These results establish PGC-1α as a driver of SCNC progression and subtype determination, highlighting metabolic vulnerabilities in SCNCs across different tissues.
Keywords: ASCL1; PGC-1a; lung cancer; oxidative phosphorylation; prostate cancer.
Conflict of interest statement
Competing interests statement:O.N.W. currently has consulting, equity, and/or board relationships with Trethera Corporation, Kronos Biosciences, Sofie Biosciences, Breakthrough Properties, Vida Ventures, Nammi Therapeutics, Two River, Iconovir, Appia BioSciences, Neogene Therapeutics, 76Bio, and Allogene Therapeutics. J.K.L. has served as a consultant for Hierax Therapeutics and has equity in, an invention licensed to, and a sponsored research agreement with PromiCell Therapeutics. O.S.S. is a co-founder and scientific advisory board member of Enspire Bio Limited Liability Company, Senergy-Bio and Capacity-Bio, and when this study was conducted, he was serving as a consultant to LUCA-Science. D.B.S. is a co-founder and consultant with Senergy-Bio and scientific advisory board member of Enspire Bio LLC. T.G.G. reports having consulting and equity agreements with Auron Therapeutics, Boundless Bio, Coherus BioSciences and Trethera Corporation. None of these companies contributed to or directed any of the research reported in this article.
Figures


Update of
-
PGC-1α drives small cell neuroendocrine cancer progression towards an ASCL1-expressing subtype with increased mitochondrial capacity.bioRxiv [Preprint]. 2024 Jul 25:2024.04.09.588489. doi: 10.1101/2024.04.09.588489. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Dec 3;121(49):e2416882121. doi: 10.1073/pnas.2416882121. PMID: 38645232 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- 01/G. Harold and Leila Y. Mathers Foundation (Mathers Foundation)
- I01 BX004651/BX/BLRD VA/United States
- I01BX006019/Veteran Affairs funds
- 01/UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research predoctoral fellowship
- 2 P30 CA016042-44/NIH (NIH)
- R01 CA267721/CA/NCI NIH HHS/United States
- 1 S10 OD026917-01A1/NIH (NIH)
- DP2 CA271301/CA/NCI NIH HHS/United States
- I01 BX006019/BX/BLRD VA/United States
- S10 OD026917/OD/NIH HHS/United States
- 01/Parker Institute for Cancer Immunotherapy (PICI)
- P50 CA092131/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- NIH T32 CA-009056/UCLA Tumor Cell Biology Training Program (USHHS) Ruth L. Kirschstein Institutional national Research Service Award
- T32 CA009056/CA/NCI NIH HHS/United States
- 01/UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Hal Gaba Director's Fund for Cancer Stem Cell Research
- 01/UCLA Dissertation Year Fellowship
- 01/UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Award
- 01/University of California-Historically Black Colleges and Universities (UC-HBCU) Initiative Fellowship provided by University of California Office of the President (UCOP).
- R01 CA222877/CA/NCI NIH HHS/United States
- R01CA222877Â/NIH R01 Grant
- P50CA092131Â/NIH UCLA SPORE in Prostate Cancer

