Trabectedin for L-Type Sarcoma: A Retrospective Multicenter Study
- PMID: 39590133
- PMCID: PMC11592548
- DOI: 10.3390/curroncol31110502
Trabectedin for L-Type Sarcoma: A Retrospective Multicenter Study
Abstract
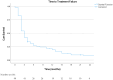
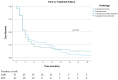
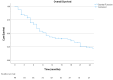
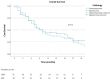
(1) Background: Metastatic L-type sarcomas (liposarcoma and leiomyosarcoma) are rare and have a poor prognosis. Trabectedin is an effective agent that can be used after anthracyclines. This study was designed to evaluate the real-life effectiveness and safety of trabectedin. (2) Methods: A retrospective multicenter study was conducted on patients who were treated with trabectedin for metastatic L-type sarcomas at ten tertiary oncology centers between 2015 and 2023. The objective response rate (ORR), disease control rate (DCR), time to treatment failure (TTF), and overall survival (OS) were evaluated in the cohort. Cox regression analysis was used to determine prognostic factors for survival. (3) Results: A total of 98 patients (52% liposarcoma and 48% leiomyosarcoma) were included in the study. The median treatment line was three (range: 1 to 6). Thirteen patients (13.3%) underwent local treatment due to oligoprogression, and dose reduction was required in seventeen patients (17.3%) due to toxicity. The ORR and DCR were 16% and 42%, respectively. The median TTF was 3 months, and the median OS was 10 months. In univariate analysis, a significantly longer median TTF was observed in patients who underwent local treatment (p = 0.008), obtained objective responses (p < 0.001), and underwent dose reduction (p = 0.002). No statistical differences were observed according to the histologic subtype and metastatic site. In the multivariate analysis for OS, it was found that obtaining an objective response was a good prognostic factor (p = 0.003), while the presence of liver metastases was associated with a poor prognosis (p = 0.016). (4) Conclusion: Trabectedin is a suitable option for L-type sarcoma after doxorubicin-based treatments. Survival was not worse in patients who underwent dose reduction. The use of local therapies simultaneously with trabectedin can be effective.
Keywords: drug toxicity; ecteinascidin 743; leiomyosarcomas; liposarcomas; targeted radiation therapy; trabectedin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ducimetière F., Lurkin A., Ranchère-Vince D., Decouvelaere A.-V., Péoc’H M., Istier L., Chalabreysse P., Muller C., Alberti L., Bringuier P.-P., et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS ONE. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. - DOI - PMC - PubMed
-
- Rao U.N., Finkelstein S.D., Jones M.W. Comparative immunohistochemical and molecular analysis of uterine and extrauterine leiomyosarcomas. Mod. Pathol. 1999;12:1001–1009. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

