Expansion of an Academic Molecular Tumor Board to Enhance Access to Biomarker-Driven Trials and Therapies in the Rural Southeastern United States
- PMID: 39590164
- PMCID: PMC11593073
- DOI: 10.3390/curroncol31110534
Expansion of an Academic Molecular Tumor Board to Enhance Access to Biomarker-Driven Trials and Therapies in the Rural Southeastern United States
Abstract
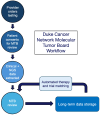
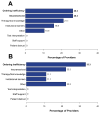
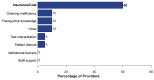
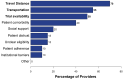
Targeting tumor-specific molecular alterations has shown significant clinical benefit. Molecular tumor boards (MTBs) connect cancer patients with personalized treatments and clinical trials. However, rural cancer centers often have limited access to MTB expertise. We established an academic-community partnership expanding our academic MTB to affiliated rural community cancer centers. We developed a centralized molecular registry of tumors (MRT) to aggregate the comprehensive genomic profiling (CGP) results and facilitate multidisciplinary MTB review. Of the 151 patients included, 87 (58%) had actionable genomic biomarkers, 42 (28%) were eligible for a targeted off-label therapy, and 27 (18%) were matched to a clinical trial. Of those with a clinical trial match, only 1 of 27 (3%) was enrolled in the identified trial. One year into implementation, community oncology providers were anonymously surveyed on persistent barriers to precision treatment utilization. The primary barriers to clinical trial enrollment were the distance to the trial center (70%), lack of transportation (55%), and lack of local trials (50%). This study offers a framework to improve access to molecular expertise, but significant barriers to the equitable use of CGP and trial enrollment persist.
Keywords: cancer disparities; comprehensive genomic profiling; molecular profiling; targeted therapy.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. J.S. declares the following conflicts: Consultant or advisory role: Abbvie, Astellas, AstraZeneca, Bayer, Beigene, Daiichi-Sankyo, Eli Lilly, GE Healthcare, GSK, Johnson and Johnson, Jazz Pharmaceuticals, Merck, Natera, Pfizer, Roche/Genentech, Regeneron, Sanofi, Taiho, Takeda, and Xilio Therapeutics. Stock options: Triumvira Immunologics. Research funding or contracted research: Abbvie, Amgen, AStar D3, Bayer, Beigene, Curegenix, Daiichi-Sankyo, Eli Lilly, Erasca, GSK, Leap Therapeutics, Novartis, Pfizer, Quanta Therapeutics, Revolution Medicines, and Roche/Genentech. R.R. declares the following conflicts: Consultant or advisory role: Pfizer and Oncohost. Research funding: Eli Lilly.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

