Amphotericin B Ocular Films for Fungal Keratitis and a Novel 3D-Printed Microfluidic Ocular Lens Infection Model
- PMID: 39590681
- PMCID: PMC11595471
- DOI: 10.3390/jof10110762
Amphotericin B Ocular Films for Fungal Keratitis and a Novel 3D-Printed Microfluidic Ocular Lens Infection Model
Abstract
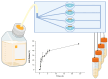
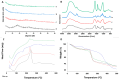
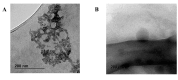
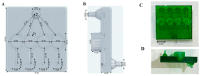
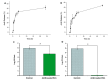
Fungal keratitis (FK), a severe eye infection that leads to vision impairment and blindness, poses a high risk to contact lens users, and Candida albicans remains the most common underpinning fungal pathogen in temperate climates. Patients are initially treated empirically (econazole 1% drops hourly for 24-48 h), and if there is no response, amphotericin B (AmB) 0.15% eye drops (extemporaneously manufactured to be stable for a week) are the gold-standard treatment. Here, we aim to develop a sustained-release AmB ocular film to treat FK with an enhanced corneal retention time. As there is a paucity of reliable in vitro models to evaluate ocular drug release and antifungal efficacy under flow, we developed a 3D-printed microfluidic device based on four chambers stacked in parallel, in which lenses previously inoculated with a C. albicans suspension were placed. Under the flow of a physiological fluid over 24 h, the release from the AmB-loaded film that was placed dry onto the surface of the wetted contact lenses was quantified, and their antifungal activity was assessed. AmB sodium deoxycholate micelle (dimeric form) was mixed with sodium alginate and hyaluronic acid (3:1 w/w) and cast into films (0.48 or 2.4%), which showed sustained release over 24 h and resulted in a 1.23-fold reduction and a 5.7-fold reduction in CFU/mL of C. albicans, respectively. This study demonstrates that the sustained delivery of dimeric AmB can be used for the treatment of FK and provides a facile in vitro microfluidic model for the development and testing of ophthalmic antimicrobial therapies.
Keywords: 3D printing; aggregation state; amphotericin B dimer; amphotericin b; keratitis; microfluidics; ocular films.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lalitha P., Shapiro B.L., Srinivasan M., Prajna N.V., Acharya N.R., Fothergill A.W., Ruiz J., Chidambaram J.D., Maxey K.J., Hong K.C., et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch. Ophthalmol. 2007;125:789–793. doi: 10.1001/archopht.125.6.789. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

