Tumor Morphology for Prediction of Poor Responses Early in Neoadjuvant Chemotherapy for Breast Cancer: A Multicenter Retrospective Study
- PMID: 39590943
- PMCID: PMC11598075
- DOI: 10.3390/tomography10110134
Tumor Morphology for Prediction of Poor Responses Early in Neoadjuvant Chemotherapy for Breast Cancer: A Multicenter Retrospective Study
Abstract
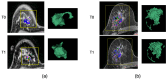
Background: This multicenter and retrospective study investigated the additive value of tumor morphologic features derived from the functional tumor volume (FTV) tumor mask at pre-treatment (T0) and the early treatment time point (T1) in the prediction of pathologic outcomes for breast cancer patients undergoing neoadjuvant chemotherapy.
Methods: A total of 910 patients enrolled in the multicenter I-SPY 2 trial were included. FTV and tumor morphologic features were calculated from the dynamic contrast-enhanced (DCE) MRI. A poor response was defined as a residual cancer burden (RCB) class III (RCB-III) at surgical excision. The area under the receiver operating characteristic curve (AUC) was used to evaluate the predictive performance. The analysis was performed in the full cohort and in individual sub-cohorts stratified by hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status.
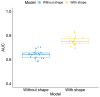
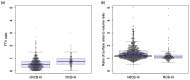
Results: In the full cohort, the AUCs for the use of the FTV ratio and clinicopathologic data were 0.64 ± 0.03 (mean ± SD [standard deviation]). With morphologic features, the AUC increased significantly to 0.76 ± 0.04 (p < 0.001). The ratio of the surface area to volume ratio between T0 and T1 was found to be the most contributing feature. All top contributing features were from T1. An improvement was also observed in the HR+/HER2- and triple-negative sub-cohorts. The AUC increased significantly from 0.56 ± 0.05 to 0.70 ± 0.06 (p < 0.001) and from 0.65 ± 0.06 to 0.73 ± 0.06 (p < 0.001), respectively, when adding morphologic features.
Conclusion: Tumor morphologic features can improve the prediction of RCB-III compared to using FTV only at the early treatment time point.
Keywords: breast cancer; magnetic resonance imaging; multicenter clinical trial; neoadjuvant therapy; residual cancer burden; tumor morphology.
Conflict of interest statement
L.J.E. is on the Blue Cross Medical Advisory Panel, is an uncompensated board member of Quantum Leap Healthcare Collaborative, and is an Investigator who initiated trial for high-risk DCIS funded by Moderna for DCIS phase 1 study. L.J.v.V. is part-time employee and stocks Agendia NV, advisor and stock options Exai Inc. N.M.H. receives institutional research funding from NIH. B.N.J. received author royalties from UpToDate, received WorldClassCME honoraria for lectures, received Medicolegal consulting payment, serves as Board of Directors for Society of Breast Imaging, Board of Trustees for RSNA R&E Foundation, and Deputy Editor for Radiology: Imaging Cancer. All authors declare no conflicts of interest regarding this study.
Figures



References
-
- Spring L.M., Fell G., Arfe A., Sharma C., Greenup R., Reynolds K.L., Smith B.L., Alexander B., Moy B., Isakoff S.J., et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-Analysis. Clin. Cancer Res. 2020;26:2838–2848. doi: 10.1158/1078-0432.CCR-19-3492. - DOI - PMC - PubMed
-
- Yee D., DeMichele A.M., Yau C., Isaacs C., Symmans W.F., Albain K.S., Chen Y.-Y., Krings G., Wei S., Harada S., et al. Association of Event-Free and Distant Recurrence-Free Survival With Individual-Level Pathologic Complete Response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: Three-Year Follow-up Analysis for the I-SPY2 Adaptively Randomized Clinical Trial. JAMA Oncol. 2020;6:1355–1362. doi: 10.1001/jamaoncol.2020.2535. - DOI - PMC - PubMed
-
- Symmans W.F., Peintinger F., Hatzis C., Rajan R., Kuerer H., Valero V., Assad L., Poniecka A., Hennessy B., Green M., et al. Measurement of Residual Breast Cancer Burden to Predict Survival after Neoadjuvant Chemotherapy. J. Clin. Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. - DOI - PubMed
-
- Provenzano E., Bossuyt V., Viale G., Cameron D., Badve S., Denkert C., MacGrogan G., Penault-Llorca F., Boughey J., Curigliano G., et al. Standardization of Pathologic Evaluation and Reporting of Postneoadjuvant Specimens in Clinical Trials of Breast Cancer: Recommendations from an International Working Group. Mod. Pathol. 2015;28:1185–1201. doi: 10.1038/modpathol.2015.74. - DOI - PubMed
-
- Bossuyt V., Provenzano E., Symmans W.F., Boughey J.C., Coles C., Curigliano G., Dixon J.M., Esserman L.J., Fastner G., Kuehn T., et al. Recommendations for Standardized Pathological Characterization of Residual Disease for Neoadjuvant Clinical Trials of Breast Cancer by the BIG-NABCG Collaboration. Ann. Oncol. 2015;26:1280–1291. doi: 10.1093/annonc/mdv161. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

