The Effectiveness of COVID-19 Vaccines During the Pre-Omicron and Omicron Periods: A Retrospective Test-Negative Case-Control Study
- PMID: 39591148
- PMCID: PMC11598972
- DOI: 10.3390/vaccines12111245
The Effectiveness of COVID-19 Vaccines During the Pre-Omicron and Omicron Periods: A Retrospective Test-Negative Case-Control Study
Abstract
Background: The aim of this study was to estimate the effectiveness of original and bivalent COVID-19 vaccines in reducing COVID-19-associated hospitalizations among the adult population of Turin, Italy.
Methods: We conducted a retrospective, test-negative, case-control study of 5768 adults aged ≥50 years who had symptoms that were consistent with COVID-19-like illness and were admitted to the hospitals of the Turin Health Unit network from 1 January 2021 to 31 January 2023. We evaluated the effectiveness of the vaccines that at the time of the study were authorized in the European Union (original/bivalent BNT162b2; original mRNA-1273; ChAdOx1-S; Ad26.COV2.S) by comparing the odds of a positive test for SARS-CoV-2 in vaccinated patients with the odds of a positive test in unvaccinated patients. The association between vaccination status, hospitalization, ICU admission and positive SARS-CoV-2 test was estimated by building multivariate adjusted logistic regression models.
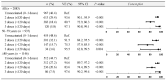
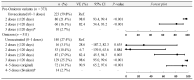
Results: During the predominance of the pre-Omicron variants, the vaccine effectiveness of two and three doses received in the last 120 days against COVID-19-associated hospitalizations was 93.6% (95% CI: 90.1 to 95.9) and 97.1% (95% CI: 90.8 to 99.1), respectively. During the predominance of the Omicron variant, the vaccine effectiveness of two and three doses was 26.6% (95% CI: -0.6 to 46.5) and 75.2% (95% CI: 68.1 to 80.7), respectively, and it rose to 88% (95% CI: 78.2 to 93.3) for four or five doses of the bivalent vaccine.
Conclusions: Our study confirms that the COVID-19 vaccines protect adult patients from hospitalizations, including the subgroup ≥80 years, also during the period of the Omicron variant's predominance.
Keywords: COVID-19; bivalent vaccine; booster; effectiveness; test-negative design; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Menni C., May A., Polidori L., Louca P., Wolf J., Capdevila J., Hu C., Ourselin S., Steves C.J., Valdes A.M., et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022;22:1002–1010. doi: 10.1016/S1473-3099(22)00146-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

