Impaired SARS-CoV-2-Specific CD8+ T Cells After Infection or Vaccination but Robust Hybrid T Cell Immunity in Patients with Multiple Myeloma
- PMID: 39591152
- PMCID: PMC11598869
- DOI: 10.3390/vaccines12111249
Impaired SARS-CoV-2-Specific CD8+ T Cells After Infection or Vaccination but Robust Hybrid T Cell Immunity in Patients with Multiple Myeloma
Abstract
Background: Multiple myeloma (MM) patients are at high risk of severe infections including COVID-19 due to an immune dysregulation affecting both innate and adaptive immune responses. However, our understanding of the immune responses to infection and vaccination in MM patients is limited. To gain more detailed insights into infection- and vaccine-elicited T cell immunity in MM, we studied the CD8+ T cell response on the single-epitope level in SARS-CoV-2 convalescent and mRNA-vaccinated MM patients.
Methods: We compared peptide/MHC class I tetramer-enriched SARS-CoV-2-specific CD8+ T cells and antibody responses in MM patients (convalescent: n = 16, fully vaccinated: n = 5, vaccinated convalescent: n = 5) and healthy controls (HCs) (convalescent: n = 58, fully vaccinated: n = 7) either after infection with SARS-CoV-2 alone, complete mRNA vaccination or SARS-CoV-2 infection and single-shot mRNA vaccination (hybrid immunity).
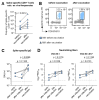
Results: MM patients have lower frequencies and a lower proportion of fully functional virus-specific CD8+ T cells compared to HCs, after both SARS-CoV-2 infection and vaccination. CD8+ T cell memory subset distribution in MM patients is skewed towards reduced frequencies of central memory (TCM) T cells and higher frequencies of effector memory 1 (TEM1) T cells. In contrast, the humoral immune response was comparable in both cohorts after viral clearance. Notably, CD8+ T cell frequencies as well as the humoral immune response were improved by a single dose of mRNA vaccine in convalescent MM patients.
Conclusions: MM patients have relative immunological deficiencies in SARS-CoV-2 immunity but benefit from hybrid immunity. These findings underline the relevance of vaccinations in this vulnerable patient group.
Keywords: COVID-19; SARS-CoV-2; T cells; adaptive immune response; immunosuppression; infection; mRNA vaccination; multiple myeloma.
Conflict of interest statement
M.K.: Advisory board GSK and Pfizer and support for attending meetings and/or travel (Janssen, GSK, Oncopeptides, Takeda, Biotest, Sobi); I.v.M.: Advisory board Pfizer, Janssen, Takeda, Amgen, Oncopeptides, BMS, GSK, Sanofi, Stemline. None of these COIs are directly related to the content of this study. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The other authors have declared that no conflicts of interest exist.
Figures



References
-
- Blimark C., Holmberg E., Mellqvist U.H., Landgren O., Bjorkholm M., Hultcrantz M., Kjellander C., Turesson I., Kristinsson S.Y. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100:107–113. doi: 10.3324/haematol.2014.107714. - DOI - PMC - PubMed
-
- Fendler A., Au L., Shepherd S.T.C., Byrne F., Cerrone M., Boos L.A., Rzeniewicz K., Gordon W., Shum B., Gerard C.L., et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: The CAPTURE study. Nat. Cancer. 2021;2:1321–1337. doi: 10.1038/s43018-021-00275-9. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

