Design of a Glycoconjugate Vaccine Against Salmonella Paratyphi A
- PMID: 39591175
- PMCID: PMC11599127
- DOI: 10.3390/vaccines12111272
Design of a Glycoconjugate Vaccine Against Salmonella Paratyphi A
Abstract
Background/objectives: Typhoid and paratyphoid fever together are responsible for millions of cases and thousands of deaths per year, most of which occur in children in South and Southeast Asia. While typhoid conjugate vaccines (TCVs) are licensed, no vaccines are currently available against S. Paratyphi A. Here we describe the design of a S. Paratyphi A conjugate.
Methods: The serovar-specific O-antigen (O:2) was linked to the CRM197 carrier protein (O:2-CRM197) and a panel of conjugates differing for structural characteristics were compared in mice and rabbits.
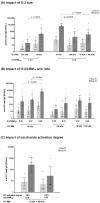
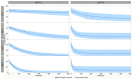
Results: We identified the O-antigen molecular size, polysaccharide to protein ratio, conjugate cross-linking, and O:2 O-acetylation level as critical quality attributes and identified optimal design for a more immunogenic vaccine.
Conclusions: This work guides the development of the O:2-CRM197 conjugate to be combined with TCV in a bivalent formulation against enteric fever.
Keywords: 1-cyano-4-dimethylaminopyridine tetrafluoroborate (CDAP); O-antigen (O:2); Salmonella Paratyphi A; acetylation; conjugate; critical quality attribute (CQA); immunogenicity; molecular size.
Conflict of interest statement
GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals; R.A., M.C., L.M., D.D.S., M.M., O.R., S.R., F.M., and C.G. are employees of the GSK group of companies.
Figures


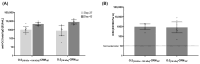
References
-
- Hasso-Agopsowicz M., Sparrow E., Cameron A.M., Sati H., Srikantiah P., Gottlieb S., Bentsi-Enchill A., Le Doare K., Hamel M., Giersing B.K., et al. The role of vaccines in reducing antimicrobial resistance: A review of potential impact of vaccines on AMR and insights across 16 vaccines and pathogens. Vaccine. 2024;42:S1–S8. doi: 10.1016/j.vaccine.2024.06.017. - DOI - PubMed
-
- Patel P.D., Liang Y., Meiring J.E., Chasweka N., Patel P., Misiri T., Mwakiseghile F., Wachepa R., Banda H.C., Shumba F., et al. Efficacy of typhoid conjugate vaccine: Final analysis of a 4-year, phase 3, randomised controlled trial in Malawian children. Lancet. 2024;403:459–468. doi: 10.1016/S0140-6736(23)02031-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

