Vaccination Against Androgen Receptor Splice Variants to Immunologically Target Prostate Cancer
- PMID: 39591176
- PMCID: PMC11599078
- DOI: 10.3390/vaccines12111273
Vaccination Against Androgen Receptor Splice Variants to Immunologically Target Prostate Cancer
Abstract
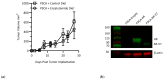
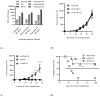
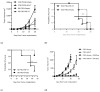
Background/Objectives: Androgen receptor (AR) expression and signaling are critical for the progression of prostate cancer and have been the therapeutic focus of prostate cancer for over 50 years. While a variety of agents have been developed to target this axis, many of these fail due to the emergent expression of AR RNA splice variants, such as AR-V7, that can signal independently of ligand binding. Other therapies, such as vaccination against prostate-specific antigens, have achieved FDA approvals but have fallen short of being incorporated as standard-of-care therapies for advanced prostate cancer. This may be due to the elevated level of immunosuppression observed in prostate cancer, which remains largely refractory to immune checkpoint blockade. Methods: We developed a vaccine targeting AR-V7, a common isoform associated with treatment resistance, and demonstrated its ability to elicit AR-V7-specific immunity and enable anti-tumor responses against AR-V7+ cancers in subcutaneous tumor models. Results: Our studies also revealed that AR-V7 expression conferred an immune suppressive phenotype that was significant in a non-AR-dependent prostate cancer model. Notably, in this model, we found that vaccination in combination with enzalutamide, an AR antagonist, suppressed these aggressive immune suppressive cancers and resulted in enhanced survival in comparison to control vaccinated and enzalutamide-treated mice. While anti-PD-1 immune checkpoint inhibition (ICI) alone slowed tumor growth, the majority of vaccinated mice that received anti-PD-1 therapy showed complete tumor elimination. Conclusions: Collectively, these results validate the importance of AR signaling in prostate cancer immune suppression and suggest the potential of AR-V7-specific vaccines as therapeutic strategies against prostate cancer, offering significant protective and therapeutic anti-tumor responses, even in the presence of androgen signaling inhibitors.
Keywords: androgen receptor; castration resistance; immune checkpoint inhibition; immune suppression; prostate cancer; vaccines.
Conflict of interest statement
Z.C.H., H.K.L. and R.D.M. are inventors of a patent to immunologically target AR isoforms by vaccination. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

