Multiparameter Flow Cytometric Analysis of the Conventional and Monocyte-Derived DC Compartment in the Murine Spleen
- PMID: 39591196
- PMCID: PMC11598974
- DOI: 10.3390/vaccines12111294
Multiparameter Flow Cytometric Analysis of the Conventional and Monocyte-Derived DC Compartment in the Murine Spleen
Abstract
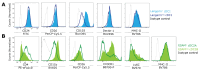
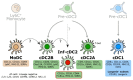
Dendritic cells (DCs) are present in almost all tissues, where they act as sentinels involved in innate recognition and the initiation of adaptive immune responses. The DC family consists of several cell lineages that are heterogenous in their development, phenotype, and function. Within these DC lineages, further subdivisions exist, resulting in smaller, less characterized subpopulations, each with its unique immunomodulatory capabilities. Given the interest in utilizing DC for experimental studies and for vaccination purposes, it becomes increasingly crucial to thoroughly classify and characterize these diverse DC subpopulations. This understanding is vital for comprehending their relative contribution to the initiation, regulation, and propagation of immune responses. To facilitate such investigation, we here provide an easy and ready-to-use multicolor flow cytometry staining panel for the analysis of conventional DC, plasmacytoid DC, and monocyte-derived DC populations isolated from mouse spleens. This adaptable panel can be easily customized for the analysis of other tissue-specific DC populations, providing a valuable tool for DC research.
Keywords: dendritic cell; dendritic cell subpopulations; macrophages; monocytes; multicolor flow cytometry; myeloid cells; spleen.
Conflict of interest statement
The authors declare that there are no commercial or financial conflicts of interest.
Figures



References
-
- Amon L., Lehmann C.H., Baranska A., Schoen J., Heger L., Dudziak D. Transcriptional control of dendritic cell development and functions. Int. Rev. Cell Mol. Biol. 2019;349:55–151. - PubMed
LinkOut - more resources
Full Text Sources

