Chlorophyll-Amended Organoclays for the Detoxification of Ochratoxin A
- PMID: 39591234
- PMCID: PMC11598794
- DOI: 10.3390/toxins16110479
Chlorophyll-Amended Organoclays for the Detoxification of Ochratoxin A
Abstract
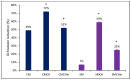
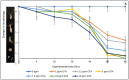
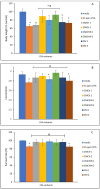
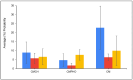
Climate change has been associated with outbreaks of mycotoxicosis following periods of drought, enhanced fungal growth, and increased exposure to mycotoxins. For detoxification, the inclusion of clay-based materials in food and drinking water has resulted in a very promising strategy to reduce mycotoxin exposure. In this strategy, mycotoxins are tightly sorbed to high-affinity clay particles in the gastrointestinal tract, thus decreasing bioavailability, uptake to blood, and potential toxicity. This study investigated the ability of chlorophyll and chlorophyllin-amended montmorillonite clays to decrease the toxicity of ochratoxin A (OTA). The sorption mechanisms of OTA binding to surfaces of sorbents, as well as binding parameters such as capacity, affinity, enthalpy, and free energy, were examined. Chlorophyll-amended organoclay (CMCH) demonstrated the highest binding (72%) and was better than the chlorophyllin-amended hydrophilic clay (59%), possibly due to the hydrophobicity of OTA (LogP 4.7). In silico studies using molecular dynamics simulations showed that CMCH improves OTA binding in comparison to parent clay in line with experiments. Simulations depicted that chlorophyll amendments on clay facilitated OTA molecules binding both directly, through enhancing OTA binding on the clay, or predominantly indirectly, through OTA molecules interacting with bound chlorophyll amendments. Simulations uncovered the key role of calcium ions in OTA binding, particularly in neutral conditions, and demonstrated that CMCH binding to OTA is enhanced under both neutral and acidic conditions. Furthermore, the protection of various sorbents against OTA-induced toxicity was carried out using two living organisms (Hydra vulgaris and Caenorhabditis elegans) which are susceptible to OTA toxicity. This study showed the significant detoxification of OTA (33% to 100%) by inclusion of sorbents. Organoclay (CMCH) at 0.5% offered complete protection. These findings suggest that the chlorophyll-amended organoclays described in this study could be included in food and feed as OTA binders and as potential filter materials for water and beverages to protect against OTA contaminants during outbreaks and emergencies.
Keywords: enterosorption; food contamination; isotherms; kinetics; molecular dynamics; mycotoxin; ochratoxin; thermodynamics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
-
- World Health Organization Food Safety. 2022. [(accessed on 5 November 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

