First-Line Lenvatinib Plus Pembrolizumab Versus Chemotherapy for Advanced Endometrial Cancer: A Randomized, Open-Label, Phase III Trial
- PMID: 39591551
- PMCID: PMC11936476
- DOI: 10.1200/JCO-24-01326
First-Line Lenvatinib Plus Pembrolizumab Versus Chemotherapy for Advanced Endometrial Cancer: A Randomized, Open-Label, Phase III Trial
Abstract
Purpose: Lenvatinib plus pembrolizumab (len + pembro) significantly improved progression-free survival (PFS) and overall survival (OS) versus chemotherapy in previously treated advanced or recurrent endometrial cancer (aEC) in the phase III Study 309/KEYNOTE-775. We report results from the phase III, randomized, open-label European Network of Gynaecological Oncological Trial-en9/LEAP-001 study (ClinicalTrials.gov identifier: NCT03884101) that evaluated len + pembro versus chemotherapy in first-line aEC.
Methods: Patients with stage III to IV or recurrent, radiographically apparent EC and no previous chemotherapy or disease progression ≥6 months after neo/adjuvant platinum-based chemotherapy were randomly assigned 1:1 to lenvatinib 20 mg once daily plus pembrolizumab 200 mg once every 3 weeks or paclitaxel 175 mg/m2 plus carboplatin AUC 6 mg/mL/min once every 3 weeks. Primary end points were PFS and OS, evaluated in the mismatch repair-proficient (pMMR) and all-comers populations. Noninferiority was assessed for OS at final analysis (FA) for len + pembro versus chemotherapy (multiplicity-adjusted, one-sided nominal alpha, .0159; null hypothesis-tested hazard ratio [HR], 1.1).
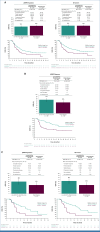
Results: Eight hundred forty-two patients were randomly assigned (len + pembro, n = 420 [pMMR population, n = 320]; chemotherapy, n = 422 [pMMR population, n = 322]). At FA (data cutoff, October 2, 2023), median PFS (95% CI) in the pMMR population was 9.6 (8.2 to 11.9) versus 10.2 (8.4 to 10.5) months with len + pembro versus chemotherapy (hazard ratio [HR], 0.99 [95% CI, 0.82 to 1.21]) and among all-comers was 12.5 (10.3 to 15.1) versus 10.2 (8.4 to 10.4) months (HR, 0.91 [95% CI, 0.76 to 1.09]; descriptive analyses). Median OS (95% CI) in the pMMR population was 30.9 (25.4 to 37.7) versus 29.4 (26.2 to 35.4) months with len + pembro versus chemotherapy (HR, 1.02 [95% CI, 0.83 to 1.26]; noninferiority P = .246, not statistically significant per multiplicity control strategy) and among all-comers was 37.7 (32.2 to 43.6) versus 32.1 (27.2 to 35.7) months (HR, 0.93 [95% CI, 0.77 to 1.12]). Grade ≥3 treatment-related adverse events occurred in 331/420 (79%) versus 274/411 (67%) treated patients.
Conclusion: First-line len + pembro did not meet prespecified statistical criteria for PFS or OS versus chemotherapy in pMMR aEC.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- Siegel RL, Giaquinto AN, Jemal A: Cancer statistics, 2024. CA Cancer J Clin 74:12-49, 2024 - PubMed
-
- European Commission : Estimates of cancer incidence and mortality in 2022, for all cancer sites. https://ecis.jrc.ec.europa.eu/
-
- Lortet-Tieulent J, Ferlay J, Bray F, Jemal A: International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst 110:354-361, 2018 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

