Functional Outcome Measures to Optimize Drug Development in Spinal and Bulbar Muscular Atrophy: Results From a Meta-Analysis of the Global SBMA Dataset
- PMID: 39591556
- PMCID: PMC11666247
- DOI: 10.1212/WNL.0000000000210088
Functional Outcome Measures to Optimize Drug Development in Spinal and Bulbar Muscular Atrophy: Results From a Meta-Analysis of the Global SBMA Dataset
Abstract
Background and objectives: Spinal and bulbar muscular atrophy (SBMA) is a rare, slowly progressive, and debilitating disease without effective treatments available. Lack of reliable biomarkers and sensitive outcome measures makes clinical research conduct challenging. The primary objective of this study was to identify clinically meaningful and statistically sensitive outcome measures enabling the evaluation of therapeutic interventions in late-stage clinical trials.
Methods: This study was a meta-analysis of SBMA patient-level data from 6 observational studies conducted in Italy, South Korea, Denmark, United Kingdom, Japan, and United States. Patients with confirmed SBMA genetic diagnosis and differing severity were enrolled following individual site protocols. Routine assessments were performed longitudinally for approximately 3 years, including one or more clinical outcomes, such as SBMA functional rating scale (SBMAFRS), 6-minute walk test (6MWT), quantitative muscle testing (QMT), and Adult Myopathy Assessment Tool (AMAT). A modified scale, m-SBMAFRS, was derived by including only lower limb and trunk subscales having lower variability and larger effect size compared with the others. Changes from baseline at follow-up time points were calculated for all measures, and percent changes using random slope models were calculated to compare clinical measure performances. A survey conducted on 196 patients by the Coordination of Rare Diseases at Sanford (CoRDS), elucidating the impact of specific disease aspects on patients' lives, was also evaluated to corroborate these research outcomes.
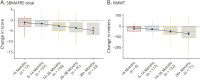
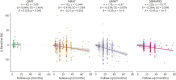
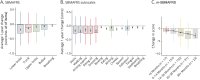
Results: This global SBMA dataset analyzed data from 278 men (mean age = 59.7 ± 10.8 years, mean disease duration = 17.7 ± 11.9 years). Patients progressed on SBMAFRS (-4.7 ± 6.2 points after 38 months with 1-year standard response mean [SRM] = 0.6) and 6MWT (distance walked decreased by -53.2 ± 87.0 meters after 26 months with 1-year SRM = 0.5). These measures showed lower variability and larger effect size than AMAT and QMT (1-year SRM = 0.1 and -0.2, respectively) and confirmed SBMA linear progression across a range of disease stages. The m-SBMAFRS also showed a significant yearly decline of 0.9 ± 1.5 points (SRM = 0.6) and more consistent performance with less variability across clinical sites. The CoRDS survey confirmed the relevance of lower limb strength and mobility, which correlated with higher quality-of-life metrics and were reported by patients as predominant disease issues.
Discussion: We generated a comprehensive global SBMA dataset, enabling the identification of sensitive functional end points for clinical trials. Possible limitations relate to data collection nuances across sites that a single study protocol could override.
Conflict of interest statement
S.B. Huggett, A.TN. Tebbenkamp and V. Viglietta are employees of Nido Biosciences who sponsored this research. C. Rinaldi, D. Jayaseelan, L. Zampedri, L. Blasi, A. Fortuna, P. Fratta, G. Soraru, S. Fenu, E. Cavalca, J. Vissing, J.S. Park, M. Kang, and D. Pareyson are participating in the Nido Biosciences Phase 2 trial. A. Alqahtani, A. Kokkinis, J. Dahlqvist, A. Bertini, C. Mariotti, C. Grunseich, T. Kawase, Y. Kishimoto, M. Katsuno, S. Yamada, A. Conte, and M. Sabatelli report no disclosures relevant to the manuscript. Go to
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
