Investigation of the effects of a new transdermal formulation of systemic diclofenac on the upper gastrointestinal mucosa in patients with low back pain: A comparative study with oral diclofenac
- PMID: 39592167
- PMCID: PMC11660207
- DOI: 10.1111/jgh.16810
Investigation of the effects of a new transdermal formulation of systemic diclofenac on the upper gastrointestinal mucosa in patients with low back pain: A comparative study with oral diclofenac
Abstract
Background and aim: Non-steroidal anti-inflammatory drugs (NSAIDs) are associated with gastrointestinal mucosal damage attributed to a topical effect of NSAIDs on the gastrointestinal mucosa after oral administration and cyclooxygenase-1 inhibition. Diclofenac sodium systemic patch (DSSP), a transdermal patch from which diclofenac sodium is absorbed through the skin to exert its effects through the circulating blood, is considered to reduce the occurrence of gastrointestinal mucosal damage compared with oral diclofenac. This study aimed to compare the effect of DSSP on the upper gastrointestinal mucosa with that of an orally administered diclofenac sodium tablet (DST).
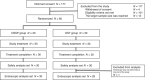
Methods: This randomized, evaluator-blinded study included Japanese patients with low back pain (LBP). The patients were administered with either DSSP (150 mg/day) or DST (75 mg/day) for 2 weeks. The primary endpoint was the incidence of gastroduodenal ulcers and/or erosions on upper gastrointestinal endoscopy after the study treatment.
Results: Thirty patients each were randomly assigned to the DSSP and DST groups. The incidence of gastroduodenal ulcers and/or erosions was 26.7% and 86.2% in the DSSP and DST groups, respectively. The difference in the incidence was -59.5% (95% confidence interval: -77.0 to -34.6). No adverse events (AEs) were observed in the DSSP group, and 20.0% (6/30 patients) reported mild AEs in the DST group (excluding ulcers and erosions).
Conclusion: DSSP is associated with a lower risk of gastrointestinal mucosal damage than DST, which has the same active ingredient but uses a different route of administration, in patients with LBP.
Keywords: (gastrointestinal) mucosal damage; diclofenac sodium; low back pain; non‐steroidal anti‐inflammatory drugs (NSAIDs); patch.
© 2024 The Author(s). Journal of Gastroenterology and Hepatology published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Figures
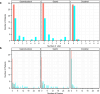
 , diclofenac sodium systemic patch (DSSP);
, diclofenac sodium systemic patch (DSSP);  , diclofenac sodium tablet (DST).
, diclofenac sodium tablet (DST).References
-
- Scheiman JM, Hindley CE. Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin. Ther. 2010; 32: 667–677. - PubMed
-
- de Leval X, Delarge J, Devel P et al. Evaluation of classical NSAIDs and COX‐2 selective inhibitors on purified ovine enzymes and human whole blood. Prostaglandins Leukot. Essent. Fat. Acids 2001; 64: 211–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

