Lateral entorhinal cortex neurons that project to nucleus accumbens mediate contextual associative memory
- PMID: 39592189
- PMCID: PMC11606517
- DOI: 10.1101/lm.054026.124
Lateral entorhinal cortex neurons that project to nucleus accumbens mediate contextual associative memory
Abstract
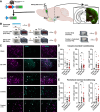
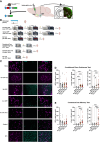
The lateral entorhinal cortex (LEC) contains glutamatergic projections that innervate the nucleus accumbens (NAc) and may be involved in the encoding of contextual associations with both positive and negative valences, such as those encountered in drug cues or fear conditioning. To determine whether LEC-NAc neurons are activated by the encoding and recall of contexts associated with cocaine or footshock, we measured c-fos expression in these neurons and found that LEC-NAc neurons are activated in both contexts. Specifically, activation patterns of the LEC-NAc were observed in a novel context and reexposure to the same context, highlighting the specific role for LEC-NAc neurons in encoding rather than the valence of a specific event-related memory. Using a combination of circuit-specific chemogenetic tools and behavioral assays, we selectively inactivated LEC-NAc neurons in mice during the encoding and retrieval of memories of contexts associated with cocaine or footshock. Chemogenetic inactivation of LEC-NAc neurons impaired the formation of both positive and negative context-associated memories without affecting the retrieval of an established memory. This finding suggests a critical role for this circuit in the initial encoding of contextual associations. In summary, LEC-NAc neurons facilitate the encoding of contextual information, guiding motivational behaviors without directly mediating the hedonic or aversive properties of these associations.
© 2024 Kuhn et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Allichon M-C, Ortiz V, Pousinha P, Andrianarivelo A, Petitbon A, Heck N, Trifilieff P, Barik J, Vanhoutte P. 2021. Cell-type-specific adaptions in striatal medium-sized spiny neurons and their roles in behavioral responses to drugs of abuse. Front Synaptic Neurosci 13: 799274. 10.3389/fnsyn.2021.799274 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
