Human Circovirus in Patients with Hepatitis, Hong Kong
- PMID: 39592266
- PMCID: PMC11616632
- DOI: 10.3201/eid3012.241114
Human Circovirus in Patients with Hepatitis, Hong Kong
Abstract
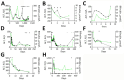
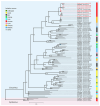
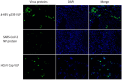
Circovirus human is a new viral species that includes the human circovirus (HCirV), which has been linked to hepatitis in immunocompromised persons. We investigated prevalence of HCirV infection in 278 patients with hepatitis and 184 asymptomatic persons using real-time PCR and sequencing assays. HCirV viremia and sequences were found in 8 (2.9%) hepatitis patients and no asymptomatic patients. Alternate causes of hepatitis (hepatitis E and cholangitis) were clearly identifiable in 2 HCirV-infected patients. HCirV could not be ruled out as a contributor to hepatitis in the remaining 6 patients, 4 of whom were immunocompromised. Persistent infections were documented in 3 patients, but only 1 had relapsing hepatitis. One HCirV patient displayed symptoms of an infectious mononucleosis-like syndrome. Isolates clustered with known HCirV strains from France and China. HCirV-derived virus-like particles bound to PLC/PRF/5 and Hep-G2 human hepatoma cells but not to lung epithelial cells, indicating hepatic tropism.
Keywords: China; Hong Kong; chronic hepatitis; circovirus; hepatitis viruses; infectious mononucleosis; viruses.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

