Eliminating chemo-mechanical degradation of lithium solid-state battery cathodes during >4.5 V cycling using amorphous Nb2O5 coatings
- PMID: 39592570
- PMCID: PMC11599776
- DOI: 10.1038/s41467-024-54331-w
Eliminating chemo-mechanical degradation of lithium solid-state battery cathodes during >4.5 V cycling using amorphous Nb2O5 coatings
Abstract
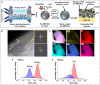
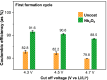
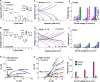
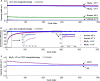
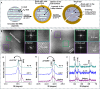
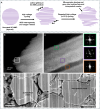
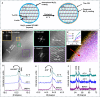
Lithium solid-state batteries offer improved safety and energy density. However, the limited stability of solid electrolytes (SEs), as well as irreversible structural and chemical changes in the cathode active material, can result in inferior electrochemical performance, particularly during high-voltage cycling (>4.3 V vs Li/Li+). Therefore, new materials and strategies are needed to stabilize the cathode/SE interface and preserve the cathode material structure during high-voltage cycling. Here, we introduce a thin (~5 nm) conformal coating of amorphous Nb2O5 on single-crystal LiNi0.5Mn0.3Co0.2O2 cathode particles using rotary-bed atomic layer deposition (ALD). Full cells with Li4Ti5O12 anodes and Nb2O5-coated cathodes demonstrate a higher initial Coulombic efficiency of 91.6% ± 0.5% compared to 82.2% ± 0.3% for the uncoated samples, along with improved rate capability (10x higher accessible capacity at 2C rate) and remarkable capacity retention during extended cycling (99.4% after 500 cycles at 4.7 V vs Li/Li+). These improvements are associated with reduced cell polarization and interfacial impedance for the coated samples. Post-cycling electron microscopy analysis reveals that the Nb2O5 coating remains intact and prevents the formation of spinel and rock-salt phases, which eliminates intra-particle cracking of the single-crystal cathode material. These findings demonstrate a potential pathway towards stable and high-performance solid-state batteries during high-voltage operation.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy8, 230–240 (2023).
-
- Wang, M. J., Kazyak, E., Dasgupta, N. P. & Sakamoto, J. Transitioning solid-state batteries from lab to market: linking electro-chemo-mechanics with practical considerations. Joule5, 1371–1390 (2021).
-
- Zhao, Q., Stalin, S., Zhao, C. Z. & Archer, L. A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater.5, 229–252 (2020).
-
- Kim, S. Y., Cha, H., Kostecki, R. & Chen, G. Composite cathode design for high-energy all-solid-state lithium batteries with long cycle life. ACS Energy Lett.8, 521–528 (2023).
Grants and funding
LinkOut - more resources
Full Text Sources

